ChapterU5: Fire: Energy , Thermodynamics, And Oxidation-reduction
Section: Chapter Questions
Problem 17RE
Related questions
Question
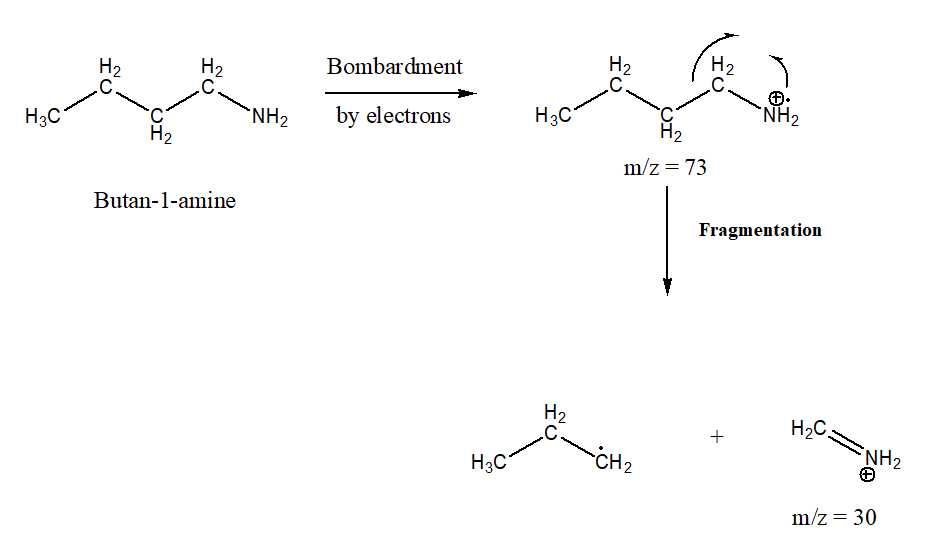
Identify the parent and propose a structure for the base peak in the mass
spectrum of butan-1-

Transcribed Image Text:100
50
10
20
30
40
50
60
70
80 90
mlz
Relative abundance
Expert Solution
Step 1
Mass spectrometry is used to detect the mass-to-charge ratio of the molecular ion. A plot of relative abundance vs m/z ratio is plotted to obtain the spectrum. A sample is bombarded with the fast-moving electrons to an ionized sample. After bombardment molecular ion is formed.
Step 2
From the spectrum, it is clear that the base peak is with m/z = 30 and the parent structure is at m/z = 73
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning



Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning
