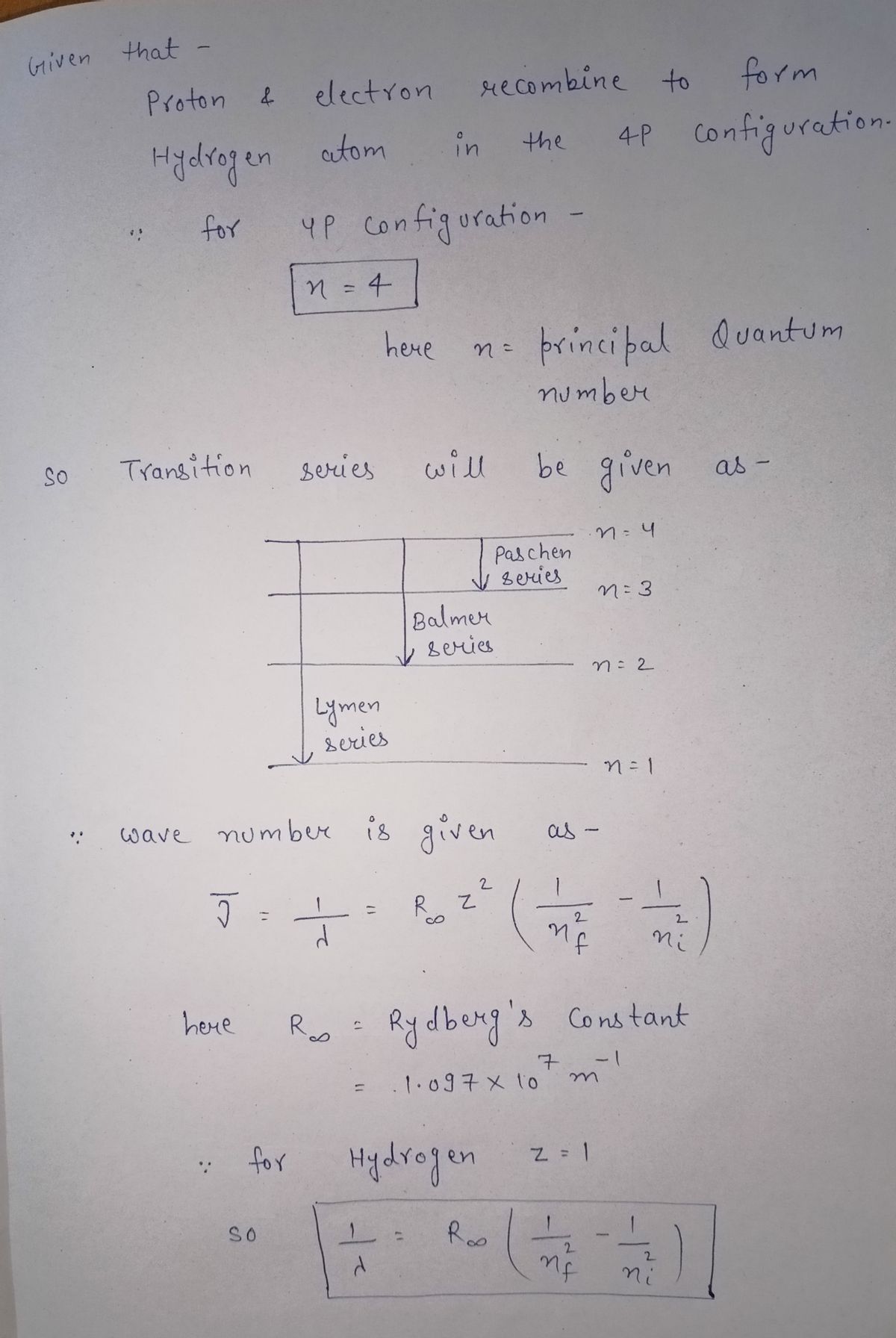
4. A proton and an electron recombine to form atomic hydrogen in its 4p state. At what wavelengths will recombination lines be observed? Label each wavelength by its standard series notation. How would the observed emissions differ if the atoms had recombined to the 4s level? (This means that the initial state is the 4p state. Determine all possible transition where a photon is emitted after the formation of the 4p state including cascading transitions. Apply selection rules)
4. A proton and an electron recombine to form atomic hydrogen in its 4p state. At what wavelengths will recombination lines be observed? Label each wavelength by its standard series notation. How would the observed emissions differ if the atoms had recombined to the 4s level? (This means that the initial state is the 4p state. Determine all possible transition where a photon is emitted after the formation of the 4p state including cascading transitions. Apply selection rules)
Related questions
Question

Transcribed Image Text:4. A proton and an electron recombine to form atomic hydrogen in its 4p
state. At what wavelengths will recombination lines be observed? Label each
wavelength by its standard series notation. How would the observed
emissions differ if the atoms had recombined to the 4s level?
(This means that the initial state is the 4p state. Determine all possible
transition where a photon is emitted after the formation of the 4p state
including cascading transitions. Apply selection rules)
Expert Solution
Step 1
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 3 images
