a reaction initially has 0.32atm I2 and 0.32ATM H2 gas. what is the equilibrium pressure of HI gas if the kp for this temp is 2.25?
Chemical equilibrium is a state in a chemical reaction when rate of formation of product or rate of forward reaction is equal to rate of consumption of reactants or the rate of backward reaction.
At equilibrium, concentration of the species becomes constant, which may or may no be equal to each other.
Initially HI gas will not exert any pressure as it has not been formed yet.
Assume that the pressure of both H2 and I2 gas reduces by P atm, at equilibrium.
Given reaction is as follows:
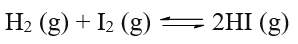
Initial Pressure exerted
at time t = 0 0.32 0.32 0 (in atm)
At equilibrium,
time t = t 0.32 - P 0.32 - P 2 * P (in atm) ; (Twice of P as 2 moles of HI are being obtained)
Step by step
Solved in 4 steps with 3 images









