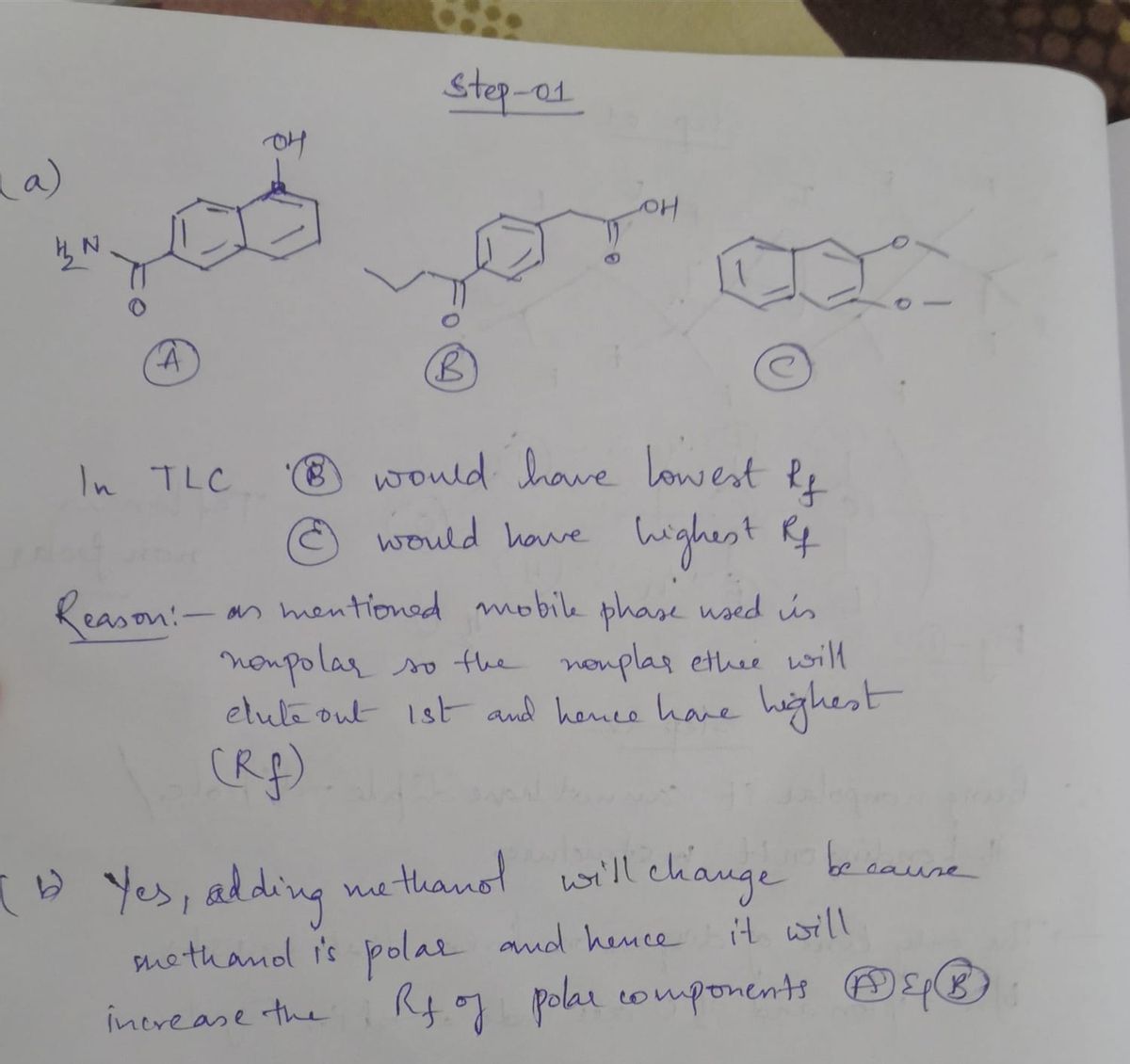
a) What would you see if you ran a Thin Layer Chromatography (TLC) on a mixture of the three solids shown below using silica gel as the stationary phase and ethyl acetate/hexanes as the mobile phase? Which solid would have the highest Rf and which would have the lowest Rf? Explain b) Would changing the mobile phase (from part a) and increasing the polarity by introducing small amount of methanol to the mobile phase change the order of Rf of the above 3 compounds? Explain c) Another type of chromatography is Reverse Phase Chromatography. In this type of chromatography, the stationary phase is non-polar and the mobile phase is polar. If a student performed Reverse Phase TLC on the mixture of 3 solids (from part a), what would be the order of Rf’s of the compounds? Which would have the lowest Rf and which would have the highest Rf?
a) What would you see if you ran a Thin Layer Chromatography (TLC) on a mixture of the three solids shown below using silica gel as the stationary phase and ethyl acetate/hexanes as the mobile phase? Which
solid would have the highest Rf and which would have the lowest Rf? Explain
b) Would changing the mobile phase (from part a) and increasing the
polarity by introducing small amount of methanol to the mobile phase change the order of
Rf of the above 3 compounds? Explain
c) Another type of chromatography is Reverse Phase Chromatography. In this type of
chromatography, the stationary phase is non-polar and the mobile phase is polar. If a
student performed Reverse Phase TLC on the mixture of 3 solids (from part a),
what would be the order of Rf’s of the compounds? Which would have the lowest Rf and
which would have the highest Rf?


Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images









