As part of an experiment to determir measures the volume of a solid sample. Her four trials yield volumes of 12.37 cm°, 12.41 cm 3, 12.39 cm 3, and cm 3. Measurements of other scientists in the lab give an average volume of 12.49 cm . Which of the following statements represents the best analysis of the data? density hava lo H Kesieion and high aCcUaCV
As part of an experiment to determir measures the volume of a solid sample. Her four trials yield volumes of 12.37 cm°, 12.41 cm 3, 12.39 cm 3, and cm 3. Measurements of other scientists in the lab give an average volume of 12.49 cm . Which of the following statements represents the best analysis of the data? density hava lo H Kesieion and high aCcUaCV
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
ChapterA: Scientific Notation And Experimental Error
Section: Chapter Questions
Problem 10P
Related questions
Question

Transcribed Image Text:00
6.0
As part of an experiment to determine the density of a new plastic developed in her laboratory, Sara Ann Dippity
measures the volume of a solid sample. Her four trials yield volumes of 12.37 cm, 12.41 cm 3, 12.39 cm 3, and 12.38
c
3. Measurements of other scientists in the lab give an average volume of 12.49 cm . Which of the following
3.
31
statements represents the best analysis of the data?
O Sara's results have low precision and high accuracy.
O Sara's results have high precision and high accuracy.
Sara's results have greater precision than accuracy.
O Sara's results have greater accuracy than precision.
O Sara has been using a faulty instrument to measure the volume.
AM
%23
%24
&
96
7.
6.
t.
alt
Expert Solution
Step 1
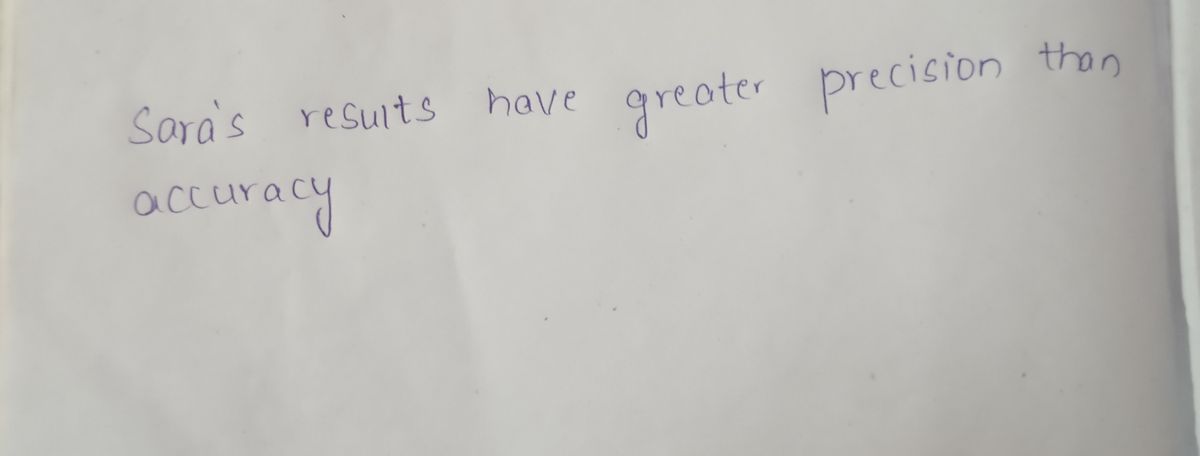
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning