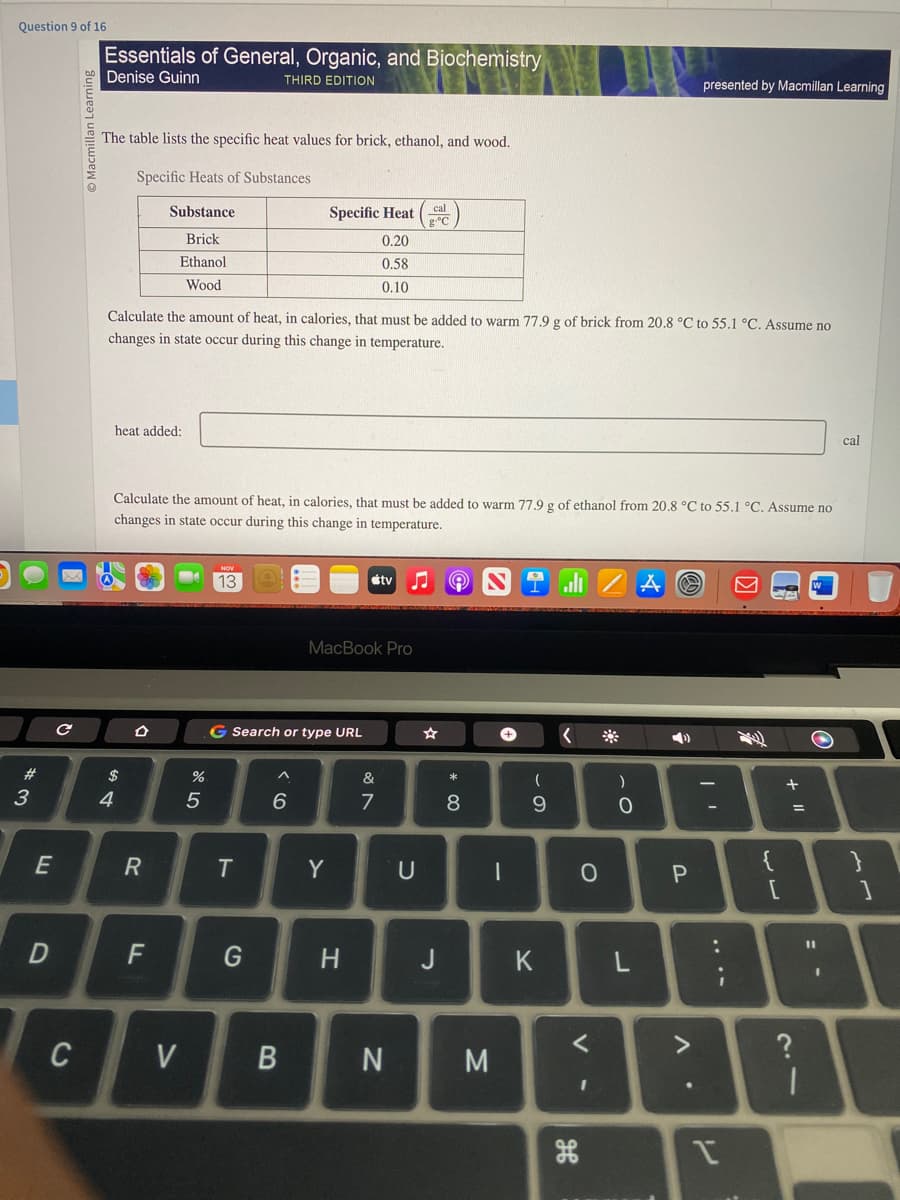
C Macmillan Learn The table lists the specific heat values for brick, ethanol, and wood. Specific Heats of Substances Substance Brick Ethanol Wood heat added: Calculate the amount of heat, in calories, that must be added to warm 77.9 g of brick from 20.8 °C to 55.1 °C. Assume no changes in state occur during this change in temperature. Specific Heat 0.20 0.58 0.10 D Calculate the amount of heat, in calories, that must be added to warm 77.9 g of ethanol from 20.8 °C to 55.1 °C. Assume no changes in state occur during this change in temperature. NOV 13 cal gºC MacBook Pro G Search or type URL #tv ♫ S Tall A cal
C Macmillan Learn The table lists the specific heat values for brick, ethanol, and wood. Specific Heats of Substances Substance Brick Ethanol Wood heat added: Calculate the amount of heat, in calories, that must be added to warm 77.9 g of brick from 20.8 °C to 55.1 °C. Assume no changes in state occur during this change in temperature. Specific Heat 0.20 0.58 0.10 D Calculate the amount of heat, in calories, that must be added to warm 77.9 g of ethanol from 20.8 °C to 55.1 °C. Assume no changes in state occur during this change in temperature. NOV 13 cal gºC MacBook Pro G Search or type URL #tv ♫ S Tall A cal
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter6: Thermochemistry
Section: Chapter Questions
Problem 68E: In a coffee-cup calorimeter, 1.60 g NH4NO3 is mixed with 75.0 g water at an initial temperature of...
Related questions
Question
Transcribed Image Text:Question 9 of 16.
#3
E
D
с
C
cmillan Learning
Essentials of General, Organic, and Biochemistry
Denise Guinn
THIRD EDITION
The table lists the specific heat values for brick, ethanol, and wood.
Specific Heats of Substances
Substance
Brick
Ethanol
Wood
4
Calculate the amount of heat, in calories, that must be added to warm 77.9 g of brick from 20.8 °C to 55.1 °C. Assume no
changes in state occur during this change in temperature.
heat added:
$
Calculate the amount of heat, in calories, that must be added to warm 77.9 g of ethanol from 20.8 °C to 55.1 °C. Assume no
changes in state occur during this change in temperature.
R
F
V
%
5
13
T
G Search or type URL
G
Specific Heat
0.20
0.58
0.10
^
6
B
MacBook Pro
Y
H
tv
&
7
N
U
J
9
* 00
8
M
+
-
(
9
K
<
O
<
H
1
)
O
O
➡
P
presented by Macmillan Learning
V
●
:
U
{
+ "1
21
=
?
11
I
cal
}
1
Transcribed Image Text:Question 9 of 16
#3
E
D
с
C
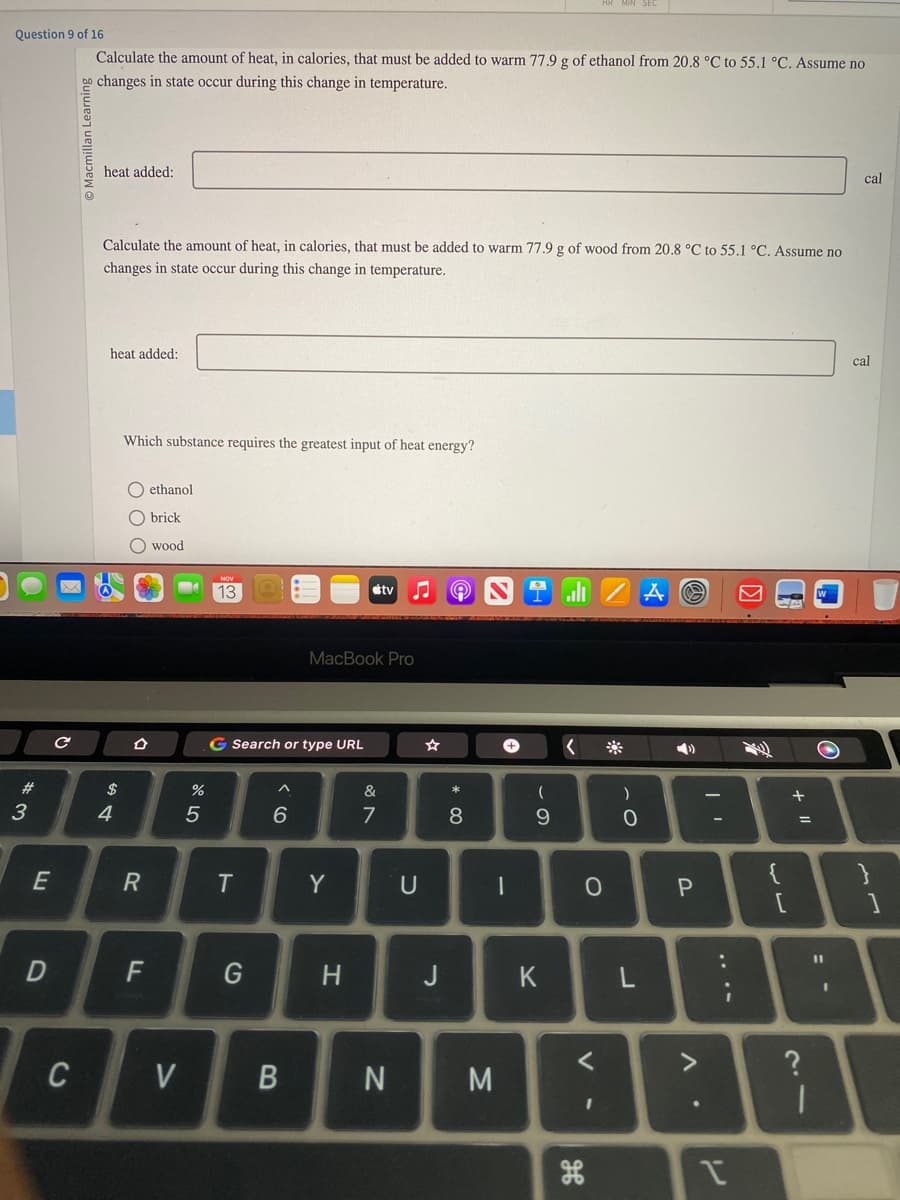
Calculate the amount of heat, in calories, that must be added to warm 77.9 g of ethanol from 20.8 °C to 55.1 °C. Assume no
o changes in state occur during this change in temperature.
Macmillan Learning
heat added:
Calculate the amount of heat, in calories, that must be added to warm 77.9 g of wood from 20.8 °C to 55.1 °C. Assume no
changes in state occur during this change in temperature.
heat added:
4
Which substance requires the greatest input of heat energy?
ethanol
brick
O wood
R
F
V
%
5
13
G Search or type URL
T
G
^
6
B
MacBook Pro
Y
H
tv
&
7
N
U
J
* 00
8
M
+
I
(
9
K
O
<
H
**
)
- O
L
A
P
V
.
I
I
{
لال
[
1 +
=
?
O
11
1
-
cal
cal
}
1
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning