Chapter30: Kinetic Methods Of Analysis
Section: Chapter Questions
Problem 30.2QAP
Related questions
Question
100%
Identify the cyclohexyl substrate which would undergo the fastest E2 with base.

Transcribed Image Text:CI
OTos
Br
CI
A
B
D
Expert Solution
Step 1
- E2 elimination is a one step concerted mechanism reaction in which dehydrohalogenation (or any other leaving group) occurs to yield the corresponding alkene.
- It is a bimolecular reaction. It is a second order reaction.
- The bond anti- to Carbon-halogen (or any other leaving group) migrates so as to give the elimination product.
Step 2
The anti- bonds in the given molecules A-D are:
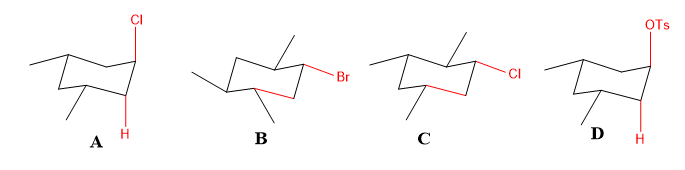
- In molecules B and C, the anti- bond cannot assist the elimination reaction as the hydrogen is not trans- to leaving group.
- In molecules A and D, the hydrogen is trans- to leaving group, so they will show fast elimination.
- Among A and D, as -OTs in D is a better leaving group, so the reaction will be fastest.
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



