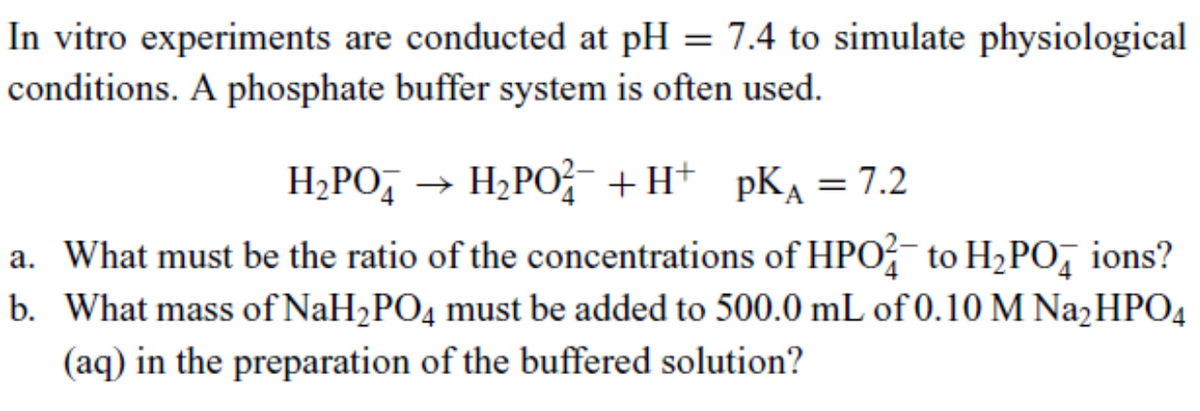
In vitro experiments are conducted at pH = 7.4 to simulate physiological conditions. A phosphate buffer system is often used. H₂PO → H₂PO¹²¯ +H+ pK₁ = 7.2 a. What must be the ratio of the concentrations of HPO to H₂POд ions? b. What mass of NaH2PO4 must be added to 500.0 mL of 0.10 M Na₂HPO4 (aq) in the preparation of the buffered solution?
Q: Some consumers would like a diet with high protein, but low fat. Which milk has the highest…
A: 3. Nonfat Cow's milk.Explanation:Introduction:Consumers often seek dietary options that offer high…
Q: Which of the following is an example of virus-encoded molecules modifying signal transduction…
A: The objective of the question is to identify which of the given options is an example of…
Q: How many extant flowering plants (angiosperms) receive pollination services from animals? 1/3…
A: Pollination is the act of transferring pollen grains from the male anther of a flower to the female…
Q: It is the holidays and you have just finished eating a large meal with your family. Now, you retire…
A: The parasympathetic nervous system (PSNS) and enteric nervous system (ENS) and their roles in…
Q: Draw an annotated graph showing the effects of light intensity on the rate of photosynthesis
A: Photosynthesis is a set of mechanisms through which photosynthetic organisms, that include most…
Q: How tablet compression should be done? I mean what is the best time for it?
A: The objective of the question is to understand the process of tablet compression and to determine…
Q: Fill in the volumes required to make the standard glucose solutions of various conentrations…
A: Tube labelVolume of 2.00mg/mL stock solutionVolume of Milli-Q waterStd 10.000320 microliterStd…
Q: QUESTION 1 In cucumbers, warty fruit (W) is dominant to smooth fruit (w) and dull fruit (D) is…
A: In the field of genetics, understanding how traits are inherited is often studied through crosses…
Q: 6. In cats, short hair, tabby, and normal (no colorpoint) are dominant traits. Long hair, no…
A: Short hair (S) and Long hair (s)Tabby (T) and no stripes (t)Normal (N, no color point) and…
Q: The leukocyte pictured above stains intensely with acidic dyes such as eosin. Which of the following…
A: The question is asking about the substance that is contained in the crystalline core of the granule…
Q: Rose plants are octoploid (octo = 8). Gametes from a rose plant contain 40 chromosomes. Indicate…
A: The correct statements are:The number of chromatids in a rose plant cell at G2 of the cell cycle is…
Q: A 90-year-old woman is brought to the emergency department 30 minutes after she fell while climbing…
A: The question is asking for the cell type that, when overactive, would most likely lead to a decrease…
Q: What is the validity of concern for potential marine extinction? Defend these concerns or lack…
A: Concern for potential marine extinction is undeniably valid given the multitude of threats…
Q: Which of these statements does NOT correctly describe hypercapnia? a. It is observed in…
A: The objective of the question is to identify the statement that does not accurately describe…
Q: What is the difference between preproinsulin and proinsulin? B. What is cleaved out of proinsulin…
A: Insulin is a small protein. It is composed of two amino acid side chains connected to each other by…
Q: ced Which of the following participates in the conduction of sound waves? 1) otolith membrane 2)…
A: The structures that participate in the conduction of sound waves include the auditory canal, malleus…
Q: The chloride shift occurs..... a. To help keep the bicarbonate equilibrium reaction moving from…
A: The chloride shift, also known as the Hamburger shift, is a physiological process occurring in red…
Q: In Aristotle’s scala naturae, the zoophytes (like sponges and sea anemones) possess: nutritive…
A: The objective of the question is to understand the classification of zoophytes (like sponges and sea…
Q: In roses, purple flower color is determined by the dominant P allele, while pp homozygotes are…
A: We're dealing with a test cross in roses, where we're uncertain about the genotype of one plant. The…
Q: Does the intake of acidic or alkaline foods affect the blood pH? a) No, the blood pH fluctuates…
A: The correct answer is: b) No, the blood pH is constantYour body has a very strong buffering system…
Q: What are the differences between sterilization, disinfection, antisepsis, degermination, and…
A: Sterilization, disinfection, antisepsis, degermination, and sanitization processes are essential for…
Q: 2 pictures of each cell -Make 2-3 short sentences about each cell (characterics, shape, size,…
A: Urinalysis, this procedure of analyzing urine, provides valuable information about a person's…
Q: A Lab Data Environment: Clean Forest Moths Released G1 G2 G3 G4 G5 Typica 250 166 259 372 521 851…
A: Phenotypic frequency corresponds to how many times a particular phenotype is observable in a given…
Q: Summarize how natural selection is the mechanism that produced a global cline of skin color. Your…
A: Natural selection is the mechanism that produced a global cline of skin color. This variation in…
Q: Skin color is directly associated with other physical and behavioral traits. True or false?
A: The question is asking whether skin color, a physical trait, is directly linked to other physical…
Q: III. Illustrate a cell with a chromosome number of N = 2 in each of the given stage of the cell…
A: The cell cycle may be a series of stages that a cell experiences because it grows and separates. A…
Q: What is the role of the liver in bilirubin metabolism? O a. The liver converts bilirubin into…
A: Hormones are biochemical messengers that help your body coordinate its various processes. Several…
Q: Yeast + hydrogen peroxide observation a. feels warm to the touch b. feels cold to the touch c.…
A: The question is asking about the type of reaction that occurs when yeast is mixed with hydrogen…
Q: Skin Deep: Nina Jablonski's Theory of Race ANTH 101 Video Response Questions. What is melanin?…
A: Melanin is a pigment that is produced by cells known as melanocytes in the skin of most animals,…
Q: Describe three characteristics of ambphibians. Think about reproductive needs, psyhical adaptations,…
A: The first characteristic of amphibians to consider is their reproductive needs. Unlike reptiles,…
Q: a) Let's say the two motif hits (CCACGAG and CCGCCAG respectively) turn out to be evolutionarily…
A: Motifs are mainly short, conserved sequence patterns that are associated with specific function of a…
Q: Cartilage does not truly repair; is this a correct statement? If yes, why?
A: Cartilage is a mature connective tissue which differentiates from mesenchyme. It consists of dense…
Q: can i have this in more detail please
A: Tinnitus is a common auditory phenomenon characterized by the perception of sound within the absence…
Q: Match the muscle type to its main characteristics Smooth muscle Cardiac muscle Skeletal muscle…
A: Match the following
Q: What do scurvy, brittle bone disease, and the hyperextensibility syndrome have in common?
A: Scurvy, brittle bone malady (osteogenesis imperfecta), and hyperextensibility syndrome (regularly…
Q: what is ONE representative figure could I make which summarises the topic of "Describe the…
A: The context for this representative figure is within the field of neuroscience, specifically…
Q: How can I structure an informational public service announcement regarding childhood vaccination,…
A: Introduction :• Start with a compelling statistic: “Did you know that childhood vaccinations prevent…
Q: Choose an example of a host adaptation that may not look advantageous at first, but was determined…
A: Good day! Kindly refer to the answer and explanation provided below. Please feel free to ask should…
Q: Which of the following is the correct sequence of events in the initiation of contraction of a…
A: The question is asking for the correct sequence of events that lead to the contraction of a skeletal…
Q: Which of the following is an incorrect statement about the inheritance of the ability to taste the…
A: Phenylthiocarbamide (PTC) is a chemical compound regarded for its bitter flavor. The capacity to…
Q: Provide a short answer for each of the questions below. For individuals homozygous for the Duffy…
A: The duffy protein is a glycoprotein expressed by duffy gene. It usually contains two antigens Fya…
Q: An enzyme is needed for the Krebs cycle. It will be made following the directions contained in a…
A: Step 1: Gene Transcription.The gene which comprises the enzyme inscribing instructions into m RNA…
Q: How do attributes and properties defined in a frame align with the broader hierarchical taxonomic…
A: Frames are utilized to hold the attributes and properties of the concept or object in question and…
Q: Which of the following is true about the pacemaker potential in the heart? a. Decreased K+ efflux…
A: The pacemaker potential, also known as the prepotential, is the slow, positive increase in voltage…
Q: Which of the following sites contains striated muscle that is not under voluntary control? A.…
A: A soft tissue that found in both animals and humans is called muscle. It is made up of actim and…
Q: You are a drug manufacturer looking to mass produce human insulin. Give a step-by-step procedure…
A: Human insulin is a peptide hormone produced by the pancreas in humans. It plays a crucial role in…
Q: A premature infant develops progressive difficulty breathing over the first few days of life.…
A: The question is asking about the type of cells in the lungs that are responsible for the synthesis…
Q: Subject: Environmental Physiology Explain the importance of the atmosphere in the biosphere in…
A: Hi student. I hope this helps you. The atmosphere plays a crucial role in the biosphere's dynamics,…
Q: A plant geneticist is examining the mode of inheritance of flower color in two closely related…
A: The objective of the question is to determine the mode of inheritance of flower color in two species…
Q: 9 7 13 14 20 20 2 6 3 110 15 16 16 S 11 12 17 18 19 19 22 23 33 15 22 21 21 27 27 28 24 29 30 30 25…
A: Within the study of evolutionary science, one critical aspect is the timing of significant occasions…
Trending now
This is a popular solution!
Step by step
Solved in 1 steps with 2 images

- Consider a buffer solution that contains 0.55 M NH2CH2CO2H and 0.35 M NH2CH2CO2Na. pKa(NH2CH2CO2H)=9.88. a. Calculate its pH. b. Calculate the change in pH if 0.155 g of solid NaOH is added to 250 mL of this solution. c. If the acceptable buffer range of the solution is ±0.10 pH units, calculate how many moles of H3O+ can be neutralized by 250 mL of the initial buffer.Describe the preparation of 2.00 L of 0.100 M glycine buffer, pH 9.0, from glycine and 1.00 M NaOH. What mass of glycine is required, and what volume of 1.00 NaOH is required? The appropriate pKa of glycine is 9.6Acetazolamide is a carbonic anhydrase inhibitor that is used to treat glaucoma. It has two acidic groups and has 2 pKb’s as shown below: What is the concentration of acetazolamide (B), in a 0.5000 M solution of acetazolamide (B)? What is the concentration of hydrogen acetazolamide (BH+) in a 0.5000 M solution of acetazolamide (B)?
- Predict the diffusivity of human serum albumin at 293 K in water as a dilute solution and compare it with experimental data. Assume the following: The molecular weight of human serum albumin, MA= 72,300 kg/kmol The viscosity of water at 293 K is 0.897 x 10-3 Pa∙s The experimental data value is 5.93 x 10-11 m2/s.Acetazolamide is a carbonic anhydrase inhibitor that is used to treat glaucoma. It has two acidic groups and has 2 pKb’s as shown below: What is the concentration of dihydrogen acetazolamide (BH22+) in a 0.5000 M solution of acetazolamide (B)? What is the concentration of hydronium ion (H+) in a 0.5000 M solution of acetazolamide (B)?Given the equation of the line, y=150x-8.1221 (cells per mL is in 10^8), what is the CFU per mL of the original suspension if the thousand-fold dilution of that suspension has an absorbance of 0.3 at 600nm? * A. 3.7 x 10^12 B. 3.7 x 10^9 C. 3.7 x 10^1 D. None of these is correct
- At 39.9ºC, a solution of ethanol (XetOH = 0.9006, P * etOH = 130.4 Torr) and isooctane (P * iso = 43.9 Torr) forms a vapor phase with YetOH = 0.6667. The total pressure is 185.9 a. Calculate the activity and the activity coefficient of each component.b. Calculate the total pressure the solution would have if it were ideal.c. Comparing the ideal pressure to the actual pressure, what does this indicate about the molecular interactions?You have been provided with stock solutions of: stock A: 0.06 M sodium pyrophosphate buffer pH 8.5 stock B: 3 M ethanol stock C: 0.015 M NAD+ stock D: milli Q water Determine the volume you will need of each solution to prepare a buffer of with a final volume of 60 mL containing 10 mM sodium pyrophosphate pH 8.5, 100 mM ethanol, 1 mM NAD+. i.e. volume of stock A = _________mL volume of stock B = _________mL volume of stock C = _________mL volume of stock D = _________mL Show your calculations to arrive at your answers.Calculate the following concentration given this
- For an acid HA, the concentrations of HA and A are 0.075 and 0.025, respectively, at pH 6.0. What is the p K a value for HA?An unknown mixture is known to contain only Ba(OH)2 (MW=171.34 g/mole) and NaOH (MW=40.0 g/mole). If the mixture is known to contain 45% by mass NaOH, and 8.0 grams of the mixture is dissolved completely in 50.0 ml of solution, answer the following. c).If 10.0 ml of a 0.2 M solution of Na2SO4 was added to the 50.0 ml solution, what would be the final concentration of Na+ in solution.In an experiment, what happens to the calculated molar mass after waiting an hour between thawing the frozen cyclohexane and adding the unknown compound, during which time some of the cyclohexane evaporated. Will the molar mass be too large, too small, or unaffected? Explain.|



