Problem 1 Propane is catalytically dehydrogenated to produce propylene by passing it over a granular solid catalyst. In parallel with the main gas-phase reaction of propane dehydrogenation, a side reaction to methane and carbon occurs: C₂H₂ →C,H, +H₂ C,H, 2CH +C A conversion of propane of 80% is achieved in the reactor. Of the propane reacting, 20% reacts according to the side reaction. Using a basis of 100 kmol.h¹ fresh feed of pure propane calculate the reactor outlet composition and flows.
Problem 1 Propane is catalytically dehydrogenated to produce propylene by passing it over a granular solid catalyst. In parallel with the main gas-phase reaction of propane dehydrogenation, a side reaction to methane and carbon occurs: C₂H₂ →C,H, +H₂ C,H, 2CH +C A conversion of propane of 80% is achieved in the reactor. Of the propane reacting, 20% reacts according to the side reaction. Using a basis of 100 kmol.h¹ fresh feed of pure propane calculate the reactor outlet composition and flows.
Oh no! Our experts couldn't answer your question.
Don't worry! We won't leave you hanging. Plus, we're giving you back one question for the inconvenience.
Submit your question and receive a step-by-step explanation from our experts in as fast as 30 minutes.
You have no more questions left.
Message from our expert:
Our experts are unable to provide you with a solution at this time. Try rewording your question, and make sure to submit one question at a time. We've credited a question to your account.
Your Question:
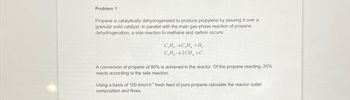
Transcribed Image Text:Problem 1
Propane is catalytically dehydrogenated to produce propylene by passing it over a
granular solid catalyst. In parallel with the main gas-phase reaction of propane
dehydrogenation, a side reaction to methane and carbon occurs:
C₂H₂ →C,H, +H₂
C,H, 2CH +C
A conversion of propane of 80% is achieved in the reactor. Of the propane reacting, 20%
reacts according to the side reaction.
Using a basis of 100 kmol.h¹ fresh feed of pure propane calculate the reactor outlet
composition and flows.
Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The
