Question 24 of 26 > The diagram represents a spontaneous reaction (AG < 0). Label the components of the diagram. Answer Bank activation energy reactants products uncatalyzed rxn catalyzed rxn AG Reation progress
Question 24 of 26 > The diagram represents a spontaneous reaction (AG < 0). Label the components of the diagram. Answer Bank activation energy reactants products uncatalyzed rxn catalyzed rxn AG Reation progress
Chapter12: Chemical Kinetics
Section: Chapter Questions
Problem 9RQ
Related questions
Question

Transcribed Image Text:I Resources
Lx Give Up?
E Feedback
Resume
O Assignment Score:
13.5%
Question 25 of 26 >
O Attempt 1
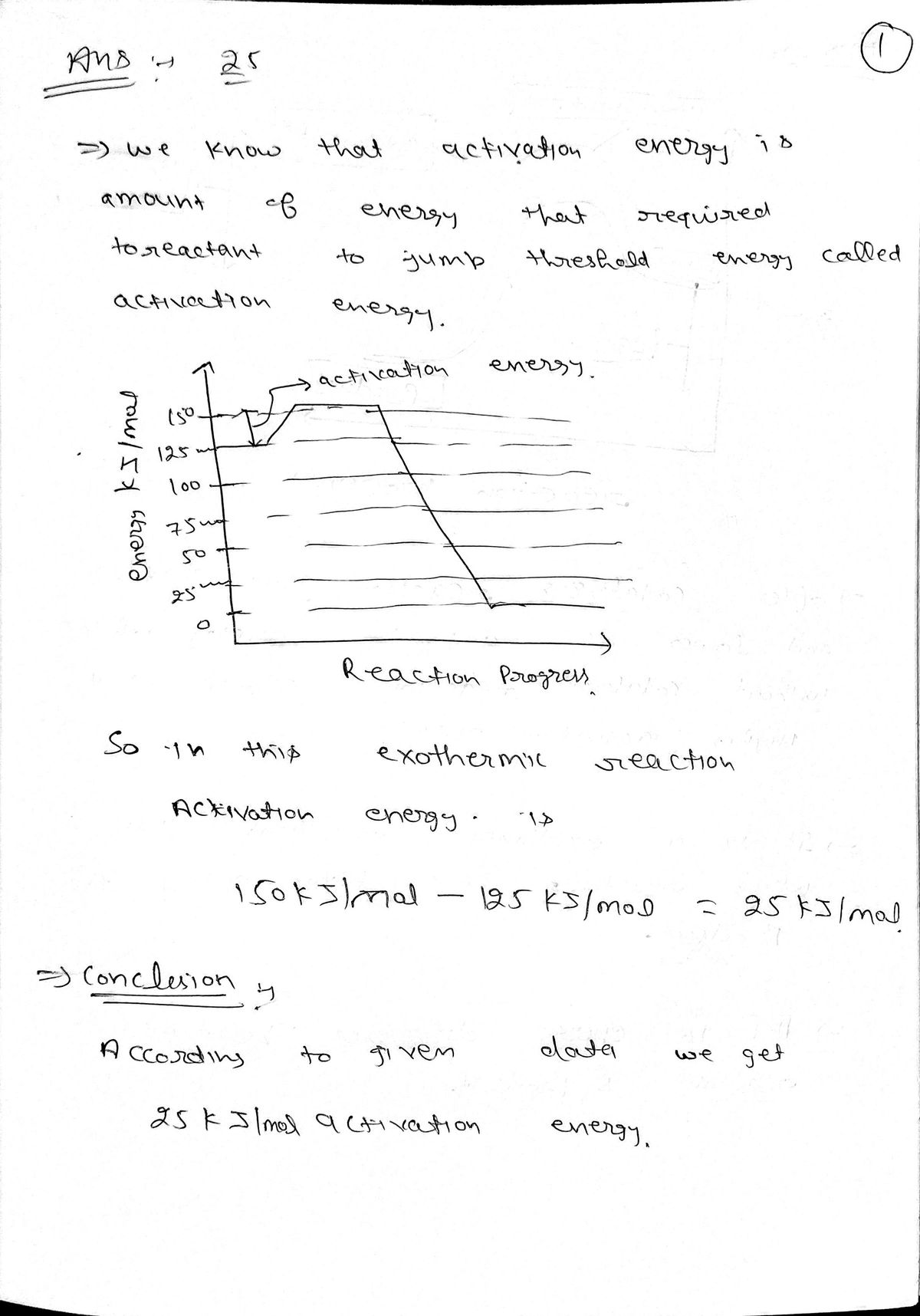
The diagram shows the energy change over the course of
a reaction.
150
Is the reaction endothermic or exothermic?
100
exothermic
50
O endothermic
What is the activation energy of the reaction?
Reaction progress
activation energy:
kJ/mol
Incorrect
acer
ctrl
Energy (kJ/mol)

Transcribed Image Text:O Assignment Score:
O Resources
GINE Up?
O Hint
13.5%
Check Answer
Question 24 of 26 >
The diagram represents a spontaneous reaction (AG < 0). Label the components of the diagram.
Answer Bank
activation energy
reactants
products
uncatalyzed rxn
catalyzed rxn
AG
Reaction progress
acer
Expert Solution
explanation question no. 25
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning