The most important contribution to the stability of a protein's conformation appears to be the: ○ A) entropy increase from the decrease in ordered water molecules forming a solvent shell around it O B) large entropy increase from ionic interactions between the ionized amino acids in a protein C) sum of the free energies of many weak interactions among the hundreds of amino acids in a protein ○ D) sum of free energies of formation of many weak interactions between polar amino acids and surrounding water E) stabilizing effect of hydrogen bonding between the carbonyl group of one peptide bond and the amino group of another
The most important contribution to the stability of a protein's conformation appears to be the: ○ A) entropy increase from the decrease in ordered water molecules forming a solvent shell around it O B) large entropy increase from ionic interactions between the ionized amino acids in a protein C) sum of the free energies of many weak interactions among the hundreds of amino acids in a protein ○ D) sum of free energies of formation of many weak interactions between polar amino acids and surrounding water E) stabilizing effect of hydrogen bonding between the carbonyl group of one peptide bond and the amino group of another
Biochemistry
9th Edition
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Chapter1: Biochemistry: An Evolving Science
Section: Chapter Questions
Problem 1P
Related questions
Question
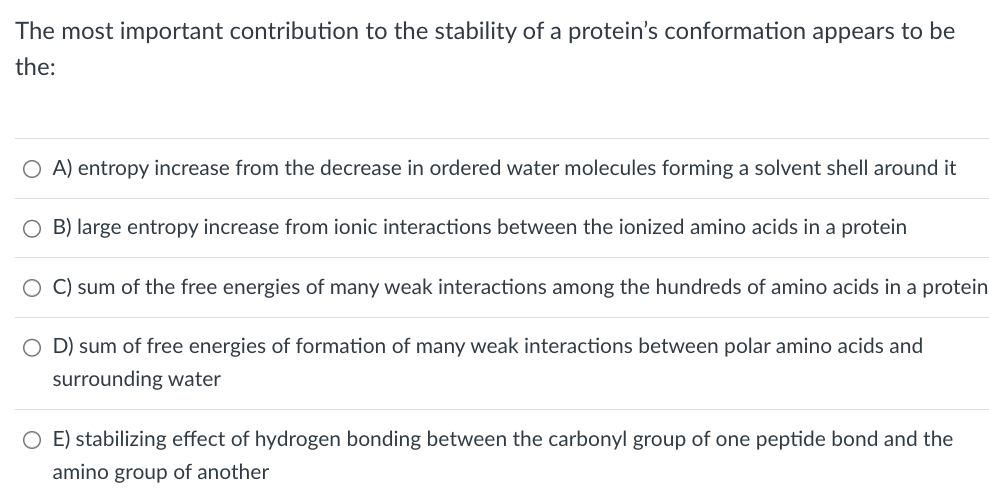
Transcribed Image Text:The most important contribution to the stability of a protein's conformation appears to be
the:
○ A) entropy increase from the decrease in ordered water molecules forming a solvent shell around it
O B) large entropy increase from ionic interactions between the ionized amino acids in a protein
C) sum of the free energies of many weak interactions among the hundreds of amino acids in a protein
○ D) sum of free energies of formation of many weak interactions between polar amino acids and
surrounding water
E) stabilizing effect of hydrogen bonding between the carbonyl group of one peptide bond and the
amino group of another
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you

Biochemistry
Biochemistry
ISBN:
9781319114671
Author:
Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:
W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:
9781464126116
Author:
David L. Nelson, Michael M. Cox
Publisher:
W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul…
Biochemistry
ISBN:
9781118918401
Author:
Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:
WILEY

Biochemistry
Biochemistry
ISBN:
9781319114671
Author:
Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:
W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:
9781464126116
Author:
David L. Nelson, Michael M. Cox
Publisher:
W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul…
Biochemistry
ISBN:
9781118918401
Author:
Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:
WILEY

Biochemistry
Biochemistry
ISBN:
9781305961135
Author:
Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:
Cengage Learning

Biochemistry
Biochemistry
ISBN:
9781305577206
Author:
Reginald H. Garrett, Charles M. Grisham
Publisher:
Cengage Learning

Fundamentals of General, Organic, and Biological …
Biochemistry
ISBN:
9780134015187
Author:
John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:
PEARSON