Q: b) OH OH
A: The question is based on the concept of organic synthesis.We need to synthesize the product using…
Q: Payalben
A: The objective of this question is to calculate the specific heat of a substance given the mass of…
Q: acid strong or weak? species present at 10-6 mol/L or greater when dissolved in water 0.0.... HCIO…
A: Answer:When added in water weak acid dissociates partially and an equilibrium exists between its…
Q: Consider the following molecule. Draw the Lewis Structure and use the drawing to fill in the…
A:
Q: consider the reaction Ag2O(s) + H2O -> 2Ag+ + 2OH- Ksp = 3.8 x 10-16 If Ag2O forms to an…
A: The given reaction is: Ag2O(s) + H2O 2Ag+ + 2OH-Ksp =
Q: Which one of the following compounds is most acidic
A: We are given different organic compounds and we have to tell which compound is most acidic. The…
Q: Draw the structures of the products, showing stereochemistry where relevant. 3. a)…
A: These are the examples of electrophilic addition reactions.
Q: What base is best for the Claisen reaction in the diagram? 1. NaOEt 2. NaOMe 3. NaOCH2Ph 4. NaOPh 5.…
A: QExplanation:The Claisen condensation is a carbon-carbon bond forming reaction that occurs between…
Q: ○ + NC ?
A: The objective of this question is to predict the product of the given organic reaction.The…
Q: What is the IUPAC name for this compound? CH3 CH3 Selected Answer: [None Given] Answers:…
A:
Q: What is the pressure in a 6.00 L tank with 65.4 grams of nitrogen gas at 385 K?
A: According to the ideal gas equation:PV = nRTHere, P = pressure of the gasV = volume of the gasn =…
Q: Name Turn in this page with your assignment. Where appropriate, you must show your work in order to…
A: 1) 2) C.3) 2.194) 4.6810 x 10-85) 2.8 x 10-56) 2.22Explanation:Step 1:Q1. B is the correct…
Q: Find the pH of 0.250 M hydrobromic acid, HBr, solution at 25o Show your work.
A: The negative of the logarithm of H+ ion concentration was defined as pH.pH = - log [H+]
Q: What is the major product of the following reaction NaCN DMSO :and mechanism CN CN B C D C, SN2 .a…
A:
Q: 2. When making the calibration curve in Part 1 of the experiment, it was assumed that the reaction…
A: See the images belowExplanation: Fe3+ = (0.001M)×(2,000μ(L/1,000 )μ (L/m)…
Q: Draw the organic product of the nucleophilic substitution reaction. Include all hydrogens atoms.…
A: In the given nucleophile substitution reaction ether compound is formed.Explanation:Approach to…
Q: a-Mn has a cubic structure with a = 0.8931 nm and a density of 7.47 g/cm³. B-Mn has a different…
A:
Q: How many grams of hy
A: The objective of this question is to calculate the mass of hydrogen chloride (HCl) in a given volume…
Q: Determine the pH of 0.082 M ammonia, NH3 (aq) and percent ionization of base.
A: The pH of the solution is calculated to be approximately pH=11.08.The percent ionization is…
Q: raw the appropriate Newman projection which leads to the major E2 ELIMINATION product. int 1: Review…
A: For the E2 elimination reaction to take place the leaving group and hydrogen should be anti-peri…
Q: An SF6 molecule has I = 3.07 x 10-45 kg m2. What is the minimum energy needed to start rotation?
A: ll
Q: A B H O Type your answers in all of the blanks and submit X₁ X N- 0 The following compound has both…
A: The objective of the question is to predict the site of reaction on a compound that has both an…
Q: Which of the choices is NOT a resonace structure of the compound above? A B C D
A: The resonance structures are the structures that represent delocalization of electrons in a molecule…
Q: Which group when attached to the benzene ring does not oxidizes by hot permanganate in the same way…
A: Given question is based on oxidation reaction.The alkyl side chain of benzene ring undergoes…
Q: Propose mechanisms consistent with the following reactions. (a) (b) (c) (d) (f) (breif Written…
A: Given reactions:We have to provide the mechanism of the reactions.N.B.: Since you have posted more…
Q: Calculate the pH at the equivalence point for the following titrations. 4A. Benzoic 50. mL of 0.12 M…
A: Given,4A) Molarity of benzoic acid = 0.12 Mvolume of benzoic acid = 50. mLMolarity of potassium…
Q: Assuming the reaction shown below takes place via an SN2 mechanism, which of the compounds shown…
A: Organic reactions can be defined as the reactions in which organic reactants react with each other…
Q: Consider the following reaction. hv Part: 0/2 Part 1 of 2 Draw the skeletal structure of all organic…
A: We have to predict th products.
Q: How many electron charge clouds, also called electron groups, surround the indicated carbon atom…
A: Answer:If degree of hybridization is 4 then atom is sp3 hybridized.If degree of hybridization is 3…
Q: Calculate the pH for 0.45 M NaOCI. The K₂ value for HOCI is 3.0 x 10-8. K₁ for OCI = [OH-] = pH =
A:
Q: Draw both additional resonance structures for the allyl -harr longleftrightarrowradical below.…
A: In the given radical species there are two consecutive double bonds. So the radical takes part in…
Q: 5. Why must you dissolve the metal samples in the fume hood? (Select the best answer.) a. The metal…
A: The question is based on experimental chemistry.We need to explain the reaction between metal and…
Q: The preparations of two aqueous solutions are described in the table below. For each solution, write…
A:
Q: Provide an acceptable name for the following compound. CH3-N-CH2CH3 Spell out the full name of the…
A: The given compound is an aniline derivative. If there are alkyl groups instead of H in -NH2 group,…
Q: Helium present inside a spherical balloon with a diameter of 7.5 m is found to be at a pressure of…
A: Given that,Diameter = 7.5 mPressure = 200 kPa.Surronding air temperature = 22 degrees Celsius.
Q: Perform the retrosynthetic analysis for the indicated bond and choose the correct synthetic…
A: We have to choose the correct option.
Q: And Option E is tsoh, h2o S ง 2 CHISH,H B HOCHUCHUCHUGH
A: When an aldehyde reacts with propane-1,3-dithiol in presence of acid catalyst it forms the…
Q: Ν 'N' SO₂R Bu3SnH, AIBN N SO₂R
A: The given reaction proceeds through the radical reactive mechanism. AIBN and Bu3SnH are used for the…
Q: draw the entire synthesis of (8-Hydroxy-2-isopropyl-3,4-dihydroisoquinolin-1 (2H)-one) as mentioned…
A: The objective of this question is to plan a multistep synthesis pathway starting from a given…
Q: ck the "draw structure" to launch the drawing utility. aw the product formed when the following…
A: There is a reagent called collins reagent that oxidizes primary alcohols to aldehydes. Reagent…
Q: QUESTION 6 Identify the salts that will not hydrolyze water. The solution will be neutral. NaCl NH41…
A: Answer:Salts of strong acid and strong base doesn't get hydrolyzed in water. So, in this problem we…
Q: Give Common name Write the common (not systematic) name of each organic molecule. structure CH3…
A: The given compound is a tertiary amine in which a nitrogen atom is attached with three alkyl groups.
Q: Each row of the table below describes an aqueous solution at about 25 °C. Complete the table. That…
A: There are different theories to explain the nature of the compound as acid or base.One of the…
Q: For each of the following acid-base reactions, calculate how many grams of each acid are necessary…
A: The objective of this question is to calculate the amount of sulfuric acid (H2SO4) required to…
Q: Is there a pattern to the way pH, amount, K, pressure increases decreases or has no change?
A: To determine or predict the changes in the equilibrium, use the Le Chatelier's…
Q: Predict the products of the reaction below. That is, complete the right-hand side of the chemical…
A: HNO3 (aq) + H2O (l)→ H3O+ (aq) + NO3- (aq)Explanation:When nitric acid (HNO3) dissolves in water…
Q: What is the major product of the following reaction? 1) B₂H6, diglyme 2) H2O2, NaOH A) OH 0 OH B) OH…
A: The reactant in given in the reaction is alkene. The reagents are given B2H6 and H2O2, NaOH. B2H6…
Q: In the laboratory a student combines 29.9 mL of a 0.426 M ammonium phosphate solution with 17.6 mL…
A:
Q: A chemist dissolved an 14.5-g sample of KOH in 100.0 grams of water in a coffee cup calorimeter.…
A: Given:Mass of KOH dissolved =14.5 gMass of water in a coffee cup calorimeter = 100.0 gTemperature…
Q: Use curved arrows to show how electrons move in this reaction. You may need to add atoms and/or…
A:
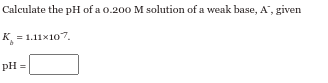
Step by step
Solved in 1 steps

- 12.62 Write the formula of the conjugate acid of each of the following bases, (a) OH-, (b) NHj, (c) CHjNHt, (d) HPO/-, (e) CO.,2’Calculate the pH for the coffee solution if [H3O+] = 2.0 x 10-5 M. Is the solution acidic, basic, or neutral?What is the pH of a solution, if there is a concentration of 0.0006 g/L of hydrogen ions?
- Ignoring significant figures, what is the pH of a solution where [OH‒] = 1.0 x 10-9 M?The hydrogen concentration of a solution is 1 x 10-14 M. What is the pH? pH = Is this an acid, base or neutral?The substance morphine is a weak nitrogenous base like ammonia.Write a net ionic equation to show how morphine, C17H19O3N, behaves as a base in water.
- If a sample of a certain solution is determined to have a [H3O+] concentration of 8.94×10^−4 moles/liter, what is its pH? Round off your answer to one decimal place.Calculate the pH of a .05 M solution of HCl(aq)?The pH of an aqueous solution that contains 3.4 x 10 -2 M NaOH is ______.What is the pOH?



