
Fundamentals of Chemical Engineering Thermodynamics (MindTap Course List)
1st Edition
ISBN: 9781111580704
Author: Kevin D. Dahm, Donald P. Visco
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 10.7, Problem 19P
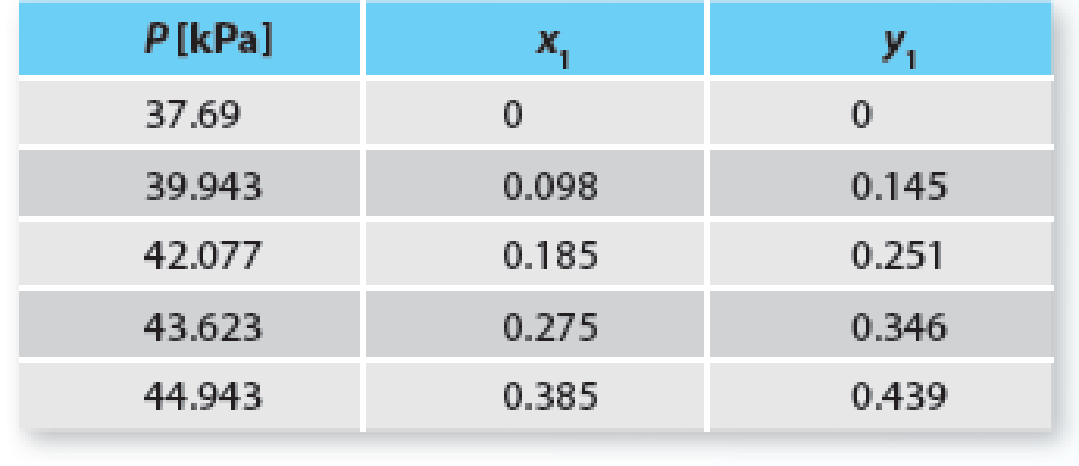
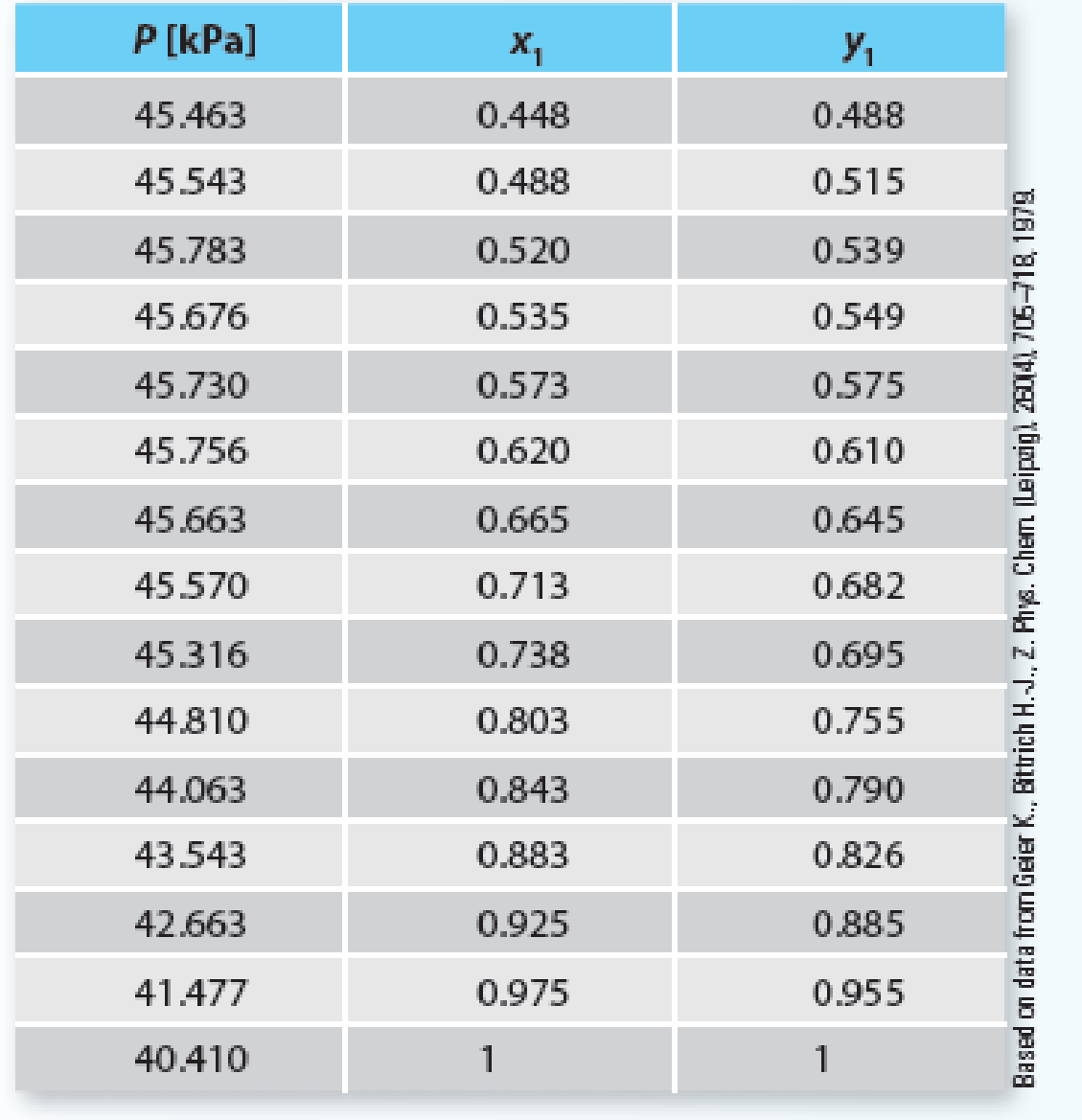
Consider the tetrahydrofuran (1) + n-hexane (2) system at 313.15 K. Using Raoult’s Law, predict the system pressure and vapor-phase composition for this mixture and provide a Pxy plot for this mixture. Compare the results of your modeling with the set of experimental data provided for this system in Table P10-19. Plot the data (as points) on the same plot as your model. Is this system properly modeled by Raoult’s Law? Why or why not?
Tetrahydrofuran
Table P10-19 Experimental data for the tetrahydrofuran (1) + n-hexane (2) system at 313.15 K.
Expert Solution & Answer
Trending nowThis is a popular solution!

Chapter 10 Solutions
Fundamentals of Chemical Engineering Thermodynamics (MindTap Course List)
Ch. 10.6 - A Pxy plot means that the pressure is constant....Ch. 10.6 - If you have a Txy plot at a given pressure and...Ch. 10.6 - What two pure component properties are you able to...Ch. 10.6 - On a Txy plot, where does the single vapor phase...Ch. 10.6 - For a mixture of n-butane (1) + n-pentane (2) at...Ch. 10.6 - Prob. 6ECh. 10.6 - Prob. 7ECh. 10.6 - For a mixture of methyl ethyl ketone (1) + water...Ch. 10.6 - For the methanol (1) + acetone (2) system at...Ch. 10.6 - Prob. 10E
Ch. 10.6 - There are four types of VLE calculations for...Ch. 10.7 - For the n-pentane (1) + methanol (2) system at...Ch. 10.7 - Consider the n-pentane (1) + methanol (2) system...Ch. 10.7 - Prob. 15PCh. 10.7 - Consider the 1-hexene (1) + n-hexane (2) system at...Ch. 10.7 - Consider the methanol (1) + ethanol (2) system at...Ch. 10.7 - Consider the n-hexane (1) + ethanol (2) system at...Ch. 10.7 - Consider the tetrahydrofuran (1) + n-hexane (2)...Ch. 10.7 - Consider the benzene (1) 1 m-xylene (2) system at...Ch. 10.7 - Prob. 21PCh. 10.7 - One hundred mol/min of an equimolar mixture of...Ch. 10.7 - One hundred mol/min of an equimolar mixture of...Ch. 10.7 - Twenty kmol/hr of an equimolar mixture of...Ch. 10.7 - A vapor mixture containing 5 moles of benzene and...Ch. 10.7 - A 50/50 (by mole) n-pentane/n-heptane liquid...Ch. 10.7 - A liquid mixture of diethyl ketone (1) and...Ch. 10.7 - A separation stream off the main reactor effluent...Ch. 10.7 - Prob. 29PCh. 10.7 - Prob. 30P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The
Homogeneous and Heterogeneous Equilibrium - Chemical Equilibrium - Chemistry Class 11; Author: Ekeeda;https://www.youtube.com/watch?v=8V9ozZSKl9E;License: Standard YouTube License, CC-BY