
Organic Chemistry (9th Edition)
9th Edition
ISBN: 9780321971371
Author: Leroy G. Wade, Jan W. Simek
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 12, Problem 12.20SP
(A true story) While organizing the undergraduate stockroom a new chemistry professor found a half-gallon jug containing a cloudy liquid (bp 100-105 °C), marked only “STUDENT PREP.” She ran a quick mass spectrum, which is printed below. As soon as she saw the spectrum (without even checking the actual mass numbers), she said, “I know what it is.”
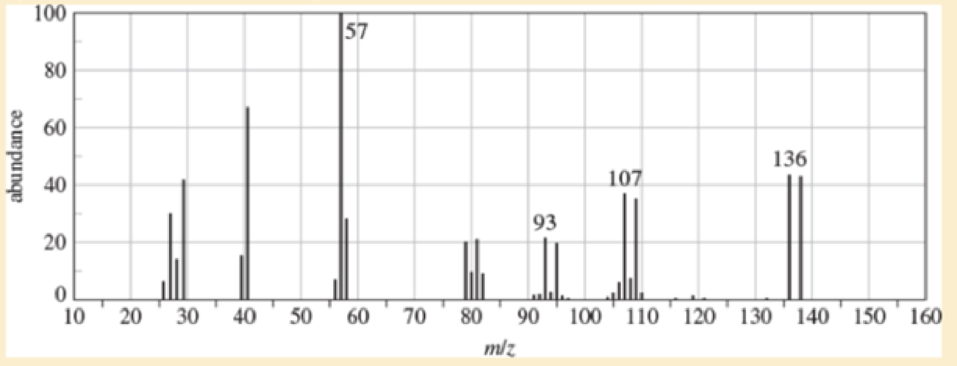
- a. What compound is the “student prep”? Any uncertainty in the structure?
- b. Suggest structures for the fragments at 136, 107, and 93. Why is the base peak (at m/z 57) so strong?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
What type of compounds/molecules can be detected using LC-MS and what mass range or molecular mass ranges can be detected using LC-MS?
In the mass spectrum of an unknown organic
compound, the molecular ion had a relative
abundance of 19.0 while the M+1 had a relative
abundance of 1.5.
Estimate the number of carbons in the molecule.
1
4
7
2
LO
5
8
carbons
3
6
9
X
C
Match the molecular ion (from electron impact mass spectrometry) with the correct compound. (Make sure to consider the isotope
pattern if necessary)
1001
50
Br
A
121
B
122
B
NH
123
123
Mass M/e
124
124
Me
C
125
125
OH
НО.
126
D
CI
127
Chapter 12 Solutions
Organic Chemistry (9th Edition)
Ch. 12.3 - Complete the following conversion table. (cm1)...Ch. 12.5 - Which of the bonds shown in red are expected to...Ch. 12.7C - For each hydrocarbon spectrum, determine whether...Ch. 12.9A - Spectra are given for three compounds. Each...Ch. 12.10 - The infrared spectra for three compounds are...Ch. 12.12 - Prob. 12.6PCh. 12.14B - Identify which of these four mass spectra indicate...Ch. 12.15A - Show the fragmentation that accounts for the...Ch. 12.15A - Show the fragmentations that give rise to the...Ch. 12.15B - Ethers are not easily differentiated by their...
Ch. 12.15C - Prob. 12.11PCh. 12 - Prob. 12.12SPCh. 12 - Prob. 12.13SPCh. 12 - All of the following compounds absorb infrared...Ch. 12 - Prob. 12.15SPCh. 12 - Four infrared spectra are shown, corresponding to...Ch. 12 - Predict the masses and the structures of the most...Ch. 12 - Prob. 12.18SPCh. 12 - Prob. 12.19SPCh. 12 - (A true story) While organizing the undergraduate...Ch. 12 - Prob. 12.21SPCh. 12 - Prob. 12.22SPCh. 12 - An unknown, foul-smelling hydrocarbon gives the...Ch. 12 - covered a synthesis of alkynes by a double...Ch. 12 - Three IR spectra are shown, corresponding to three...Ch. 12 - Prob. 12.26SPCh. 12 - Prob. 12.27SPCh. 12 - Prob. 12.28SPCh. 12 - The ultimate test of fluency in MS and IR is...Ch. 12 - Prob. 12.30SPCh. 12 - Consider the following four structures, followed...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Practice Problem 1.22 Which of the following alkenes can exist as cis-trans isomers? Write their structures. Bu...
Organic Chemistry
Practice Exercise 1
Which of the following factors determines the size of an atom? a. the volume of the nucleus...
Chemistry: The Central Science (14th Edition)
Practice Problem ATTEMPT
Write the rate expressions for each of the following reactions:
(a)
(b)
(c)
Chemistry
Give the IUPAC name for each compound.
Organic Chemistry
Real walls are never totally adiabatic. Use your experience to order the following walls in increasing order wi...
Thermodynamics, Statistical Thermodynamics, & Kinetics
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Rank these confirmations from least to most stable (le H. エ H,C. エ H. Br Br. CH, H,C CH3 HH,C H,C H. H. %3D H. Br |< ||< |I| III< ||arrow_forward14. Shown below are the mass spectra (without data tables) of four molecules. (The structure of each molecule is shown on the spectrum.) Mark the [M] peak for each, and indicate the molecular weight of the molecule. Relative Intensity Relative Intensity 100 80 60 40- 20 0-mm 10 100- 80 a. 20- 115-14-5501 0-mm 10 20 b. =0 20 30 30 40 40 50 50 60 m/z 60 m/z 70 70 80 80 90 100 90 100 Relative Intensity Relative Intensity 100- 80 40 20 0 100 80 40 10 20- 15-NU-5580 15 20 25 115--555 ont 10 20 30 30 35 40 45 50 55 m/z 40 50 m/z 60 60 70 65 70 75 80 Based on the five mass spectra you have seen, describe how one might identify the [M]* peak (molecular ion peak) on the mass spectrum of an unknown compound.arrow_forward2clar ISTRY EXAM A Chemistry student performed an experiment on the preparation and identification of an ester. He was given solutions of a carboxylic acid and an alcohol. He then prepared the ester as per laboratory instruction and identified the odour of the ester as a pear flavour. A carboxylic acid has empirical formula CH,O, and its structure is determined by using various spectroscopic techniques. (a) The mass spectrum for this carboxylic acid molecule is given below. 100- 80- 60- relative intensity 40- 20- 10 15 20 25 30 35 40 45 50 55 60 65 70 75 mass/charge (m/e) (i) State the relative molecular mass of this molecule; hence determine its molecular formula. (ii) Draw the structural formula of the carboxylic acid and give its systematic name. ark (b) Use the information provided by its Infrared spectrum below to answer the following questions 100arrow_forwardMatch the molecular ion (from electron impact mass spectrometry) with the correct compound. (Make sure to consider the isotope pattern) 100 120 Br A 121 x B NH ន 122 Mass M/e 123 123 Me 124 124 OH но ма 125 D 126arrow_forwardBy knowing the natural abundance of minor isotopes, it's possible to calculate the relative heights of M+ and M+1 peaks. If natural abundances are 12C - 98.9% and 13C - 1.10%, what are the relative heights, to the nearest 0.1%, of the M+ and M+1 peaks in the mass spectrum of benzene, C6H6? Ignore the contributions of isotopes like 2H (deuterium; 0.015% natural abundance) and 17O (0.04% natural abundance) that are small.The relative heights are, in order of increasing mass: 100 to ____arrow_forwardWhat is not true about mass spectrometry? Question 13 options: Only the molecular ion and cationic fragments are deflected, and they are then separated by their mass-to-charge ratio (m/z) It is used to determine the molecular weight and molecular formula of a compound. In a mass spectrometer, a compound is converted into ions, which are then separated by a magnetic field. In a mass spectrometer, a compound is first vaporized and then bombarded with electromagnetic radiation which generates a radical cation that is symbolized by (M)+• A molecular ion is often very unstable and susceptible to fragmentationarrow_forwardDraw the molecular ion (M+) for this molecule formed in the mass spectrometer. Br: -e 4arrow_forward100 <-this tall line is the border of the spectrum. It is not a peak from the 20 100 125 m/z compound. The compound which gave rise to this mass spectrum is most likely to: O contain bromine O contain only carbon and hydrogen O contain only carbon, hydrogen, and oxygen O contain chlorine Relative Ihtensityarrow_forwardThe compound 3-methyl-1-butanethiol, (CH3)₂CHCH₂CH₂SH, is found in the defensive secretion, or "spray," of striped skunks. Draw the skeletal structure. Draw hydrogen atoms attached to the sulfur, where applicable. Select G S Draw C H S Templates More Erase Q2Qarrow_forwardHow is mass spectrometry used to determine drugs in blood serum?arrow_forwardWhat is the molar mass of the substance whose mass spectrum is shown below? Relative Intensity 100- 80- 60 40- 20- 0 10 O:43 g/mol O 15 g/mol 60 g/mol © 29 g/mol 45 g/mol 19-4-194 15 20 40 45 50 m/z 60 70 75arrow_forwardDraw the molecular mass fragments for the following m/z rations. Show all the atoms (including) all the hydrogens clearly. CI m/z 105 m/z 139 m/z 141arrow_forwardarrow_back_iosSEE MORE QUESTIONSarrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Mass Spectrometry; Author: Professor Dave Explains;https://www.youtube.com/watch?v=hSirWciIvSg;License: Standard YouTube License, CC-BY