
Organic Chemistry (9th Edition)
9th Edition
ISBN: 9780321971371
Author: Leroy G. Wade, Jan W. Simek
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 12.5, Problem 12.2P
Which of the bonds shown in red are expected to have IR-active stretching frequencies?
- (a) H—C≡C—H
- (b) H—C≡C—H
- (c) H—C≡C—CH3
- (d) H3C—C≡C—CH3
- (e) H3C—C≡C—CH3
- (f) H3C—CH3
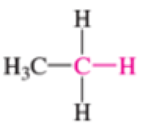
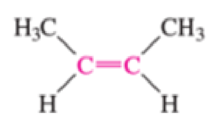
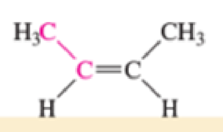
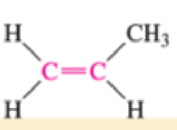
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
5. What are the spin system of the following compounds:
(a)
Cl₂CHCH2CHC₁₂
(b) CF3C=C-H
(c)
CI-
H
H
H
H
-Br
(d)
H3C
H
CH3
Q 1(a)
Methane, CH4 is a tetrahedral molecule and does not exhibit a permanent dipole
moment (i.e. it has a dipole moment of 0). Nonetheless, in microwave spectroscopy
methane does exhibit a weak, but measurable absorbance. Explain why you think this
is the case?
Q 1(b)
The diatomic molecule 12C32S has been detected in interstellar gas clouds by
microwave spectroscopy. The masses of the two atoms are C = 12.00 amu and S=
31.972 amu and 12C32S has an equilibrium bond length of 1.534 Å.
Predict which rotational transition in 12C32S will have the greatest population at a
temperature of 85 K.
Without doing the calculation, which member of each pair do you expect to occur at the higher frequency?
(a) C=O or
(b)
(c)
C=C stretching
C=O
C=C
C=O or
C-O stretching
C=O
-0
C=C or
C-O stretching
O O
C=C
C=O
(d) C-H or
C-C1 stretching
C-H
C-CI
Chapter 12 Solutions
Organic Chemistry (9th Edition)
Ch. 12.3 - Complete the following conversion table. (cm1)...Ch. 12.5 - Which of the bonds shown in red are expected to...Ch. 12.7C - For each hydrocarbon spectrum, determine whether...Ch. 12.9A - Spectra are given for three compounds. Each...Ch. 12.10 - The infrared spectra for three compounds are...Ch. 12.12 - Prob. 12.6PCh. 12.14B - Identify which of these four mass spectra indicate...Ch. 12.15A - Show the fragmentation that accounts for the...Ch. 12.15A - Show the fragmentations that give rise to the...Ch. 12.15B - Ethers are not easily differentiated by their...
Ch. 12.15C - Prob. 12.11PCh. 12 - Prob. 12.12SPCh. 12 - Prob. 12.13SPCh. 12 - All of the following compounds absorb infrared...Ch. 12 - Prob. 12.15SPCh. 12 - Four infrared spectra are shown, corresponding to...Ch. 12 - Predict the masses and the structures of the most...Ch. 12 - Prob. 12.18SPCh. 12 - Prob. 12.19SPCh. 12 - (A true story) While organizing the undergraduate...Ch. 12 - Prob. 12.21SPCh. 12 - Prob. 12.22SPCh. 12 - An unknown, foul-smelling hydrocarbon gives the...Ch. 12 - covered a synthesis of alkynes by a double...Ch. 12 - Three IR spectra are shown, corresponding to three...Ch. 12 - Prob. 12.26SPCh. 12 - Prob. 12.27SPCh. 12 - Prob. 12.28SPCh. 12 - The ultimate test of fluency in MS and IR is...Ch. 12 - Prob. 12.30SPCh. 12 - Consider the following four structures, followed...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which would you expect to have higher frequency C=O stretching vibration? H ہو H H H —F Farrow_forwardor each of the following molecules, construct the MOS from the 2p, atomic orbitals perpendicular to the plane of the carbon atoms. (a) Cyclobutadiene HC=CH НС—СН HC=CH НС —СH (b) Allyl radical H H H H C=C •C-C, H C-H H C-H H H Indicate which, if any, of these orbitals have identical ener- gies from symmetry considerations. Show the number of electrons occupying each w MO in the ground state, and indicate whether either or both of the molecules are para- magnetic. Assume that the C atoms in the allyl radical are all sp? hybridized. Activate io to Sarrow_forwardAssign the configuration Z or E to each double bond, where appropriate, in the following molecules. (a) (b) (c) Br Br NO2arrow_forward
- A chair structure of a trisubstituted cyclohexane is shown below. Determine which of the following 2D representations matches the chair structure. CH3 CH3 H3C- CH3 A) I II CH3 CH3 CH3 B) I| CH C) II CH3 IV CH, CH3 CH3 D) IVarrow_forwardAssign the correct constitutional isomer to this spectrum (C5H12O)arrow_forwardThe absorption pattern in the UV/VIS region corresponds to (a) bond vibrations b) valance electron transitions =) molecular rotations E) nuclear spin 0000 a an IR spectrumarrow_forward
- 13C NMR spectroscopy provides valuable information about the environments of a molecule's carbon atoms. Since carbon atoms are often connected to hydrogen atoms, which could split the carbon signal through spin-spin coupling, the coupling between C and H is often "turned off" through the use of broadband decoupling, causing each C signal to appear as a singlet. Draw an isomer of C5H11Cl that would be expected to have four resonances in its 13C NMR spectra.arrow_forwardA typical 1"B – 'H bond has a stretching frequency of 2400 cm1. Based on your understanding of isotopic effects on vibrational frequencies, and their connection to bond strength, rank the B - H bonds from weakest to strongest. Consider the most common isotopes of the B- H bonds highlighted below. Make sure to explain your ranking. 10В — 1н, 10В — ?н, 11в — 1Н, 11В - нarrow_forwardThe degree of unsaturation, or index of hydrogen deficiency, is the number of pi bonds plus rings in a molecule. Specify the degree of unsaturation (index of hydrogen deficiency) of the following formulas: (a) C9H12 (b) C,H40| (c) C,H,N2arrow_forward
- 7. Assign E or Z configuration to the following molecules: (A) (B) (C) (D) (E) I = Z; II = E I=E: II = Z I= E; II = E I=Z; II = Z I= E; II is neither E nor Z OH I II C1arrow_forward8. Which of the following pairs of compounds is likely to absorb radiation at the longer wavelength and with greater intensity? (a) CH3CH2CO₂H or CH2=CHCO₂H (b) CH3CH=CHCH=CHCH3 or CH3C = C—C = CCH3 OCH3 (c) or CH3arrow_forward2. Which of the following compounds would you expect to have the highest infrared absorption frequency C-X bond? Bubble in your answer completely. O Br O O Oarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
IR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=_TmevMf-Zgs;License: Standard YouTube License, CC-BY