
Concept explainers
Interpretation:
A VB picture of the
Concept introduction:
Some chemical and structural features of molecules are unaccounted for by VB theory, most notably electron delocalization, or resonance, over three or more nuclei. The VB theory picture of a species that has resonance is inaccurate because it represents a single resonance structure, not the hybrid.
Answer to Problem 14.1P
The VB picture of the
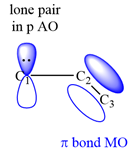
The structure of the resonance hybrid of allyl anion is
Explanation of Solution
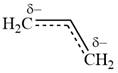
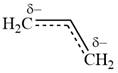
All three carbon atoms in the allyl anion are
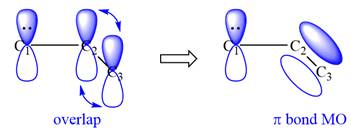
This corresponds to a double bond between C2 and C3, with a
The VB theory picture of a given species represents a single resonance structure and not the hybrid.
Want to see more full solutions like this?
Chapter 14 Solutions
EBK GET READY FOR ORGANIC CHEMISTRY
- Please answer this NEATLY, COMPLETELY, and CORRECTLY for an UPVOTE. Determine the hybridization around C2 and nitrogen atoms. What is the expected bond angle around C2 atoms? Identify the type of bonds (σ or π) and the overlapping orbitals that form bonds A and B.arrow_forwardTag all the sp3 hybridized carbon atoms in this molecule. If there are none, please check the box below. H :0: H-CC-H | H There are none. X Sarrow_forwarddoes this compound contain an sp2-hybridized carbon atom?arrow_forward
- Diagram the pi molecular orbitals for the ion below. This drawing should be top view. + CH3 H ШІНarrow_forwardPlease answer this NEATLY, COMPLETELY, and CORRECTLY for an UPVOTE. Determine the hybridization around C1, C2 and oxygen atoms. What is the expected bond angle around C1, and C2 atoms? Identify the type of bonds (σ or π) and the overlapping orbitals that form bonds A, B, and C.arrow_forwardWhich is a correct statement about the three planar aromatic ring compounds, C4H42, C5H5, C6H6? O They are all incorrect. O They are all correct. O C4H42 has a quadruply degenerate second energy level. O C5H5 has a 5-degenerate second energy level. O CoHo has a 6-fold degenerate second energy level.arrow_forward
- (d) The valence orbitals of two identical atoms are shown in the figure below left. Construct a labelled energy level diagram showing the molecular orbitals formed between these two atoms. What would be the consequences of significantly smaller energy differences between the atomic orbitals as illustrated below right? Energy р S р S Energy р S -Sarrow_forwardAccording to valence bond theory, what is the total number of sp2 hybridized orbitals on all the atoms in formaldehyde, CH2O? Sketch and explain why. According to valence bond theory, how many lone pairs of electrons reside in sp hybridized orbitals in formaldehyde? explain why?arrow_forwardTag all the sp hybridized carbon atoms in this molecule. If there are none, please check the box below. H-O H | -C-H | H There are none. Xarrow_forward
- Draw the valence bond diagram representing chemical bonding in the molecule of HCONH2 , showing atomic and hybridized orbitals as boxes, valence electrons as arrows in the boxes, and chemical bonds as lines connecting the boxes. Note that the lone pair on N occupies an unhybridized 2p orbital due to the contribution of resonance structure.arrow_forwardDraw the delocalized molecular orbitals for the following molecule. Are both bonds of the triple bond involved in the delocalized orbitals?arrow_forwardUsing cartoon representations, draw a molecular orbital mixing diagram for a CO bond. In your picture, consider the relative energies of C and O and how this changes the resulting bonding and antibonding molecular orbitals relative to a CC bond.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning

