
Organic Chemistry - Standalone book
10th Edition
ISBN: 9780073511214
Author: Francis A Carey Dr., Robert M. Giuliano
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 15.4, Problem 3P
Lithium diisopropylamide is often used as a strong base in
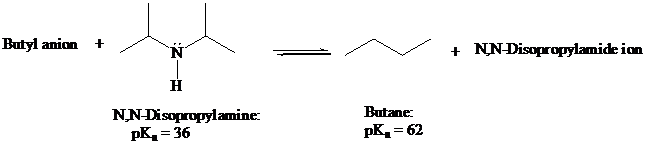
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
An analytical chemist is titrating 173.4 mL of a 0.6400 M solution of trimethylamine ((CH,) N with a 0.8100 M solution of
HNO3.
The p K,
of
3
trimethylamine is 4.19. Calculate the pH of the base solution after the chemist has added 59.0 mL of the HNO, solution to it.
3.
Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of HNO,
solution added.
Round your answer to 2 decimal places.
o Calculate the pH of a 0.132 M solution of ethylenediamine (H₂NCH₂CH₂NH₂). The pKa values for the acidic form of
ethylenediamine (H₂NCH₂CH₂NH³) are 6.848 (pKal) and 9.928 (pK a2).
pH =
Calculate the concentration of each form of ethylenediamine in this solution at equilibrium.
[H₂NCH₂CH₂NH¹] =
[H NCH₂CH₂NH₂ ] =
M
M
Write the net ionic equation for the equilibrium that is established when calcium cyanide is dissolved in water.
Chapter 15 Solutions
Organic Chemistry - Standalone book
Ch. 15.1 - Each of the following organometallic reagents will...Ch. 15.3 - Write equations showing how you could prepare...Ch. 15.4 - Lithium diisopropylamide is often used as a strong...Ch. 15.5 - Write the structure of the organic product of each...Ch. 15.7 - Prob. 5PCh. 15.8 - Prob. 6PCh. 15.9 - Prob. 7PCh. 15.9 - Like nickel, iron reacts with carbon monoxide to...Ch. 15.9 - Prob. 9PCh. 15.9 - What is the oxidation state of manganese in the...
Ch. 15.9 - Prob. 11PCh. 15.10 - Prob. 12PCh. 15.10 - Prob. 13PCh. 15.11 - Give the structure including stereochemistry of...Ch. 15.11 - Prob. 15PCh. 15.12 - Homogeneous catalytic hydrogenation of the...Ch. 15.12 - Prob. 17PCh. 15.13 - What alkenes are formed from 2-pentene by olefin...Ch. 15.13 - The product of the following reaction was isolated...Ch. 15 - Suggest appropriate methods for preparing each of...Ch. 15 - Prob. 21PCh. 15 - Predict the principal organic product of each of...Ch. 15 - Prob. 23PCh. 15 - Predict the principal organic product of each of...Ch. 15 - Prob. 25PCh. 15 - A different stereoisomer of...Ch. 15 - Prob. 27PCh. 15 - Using phenyllithium and any necessary organic or...Ch. 15 - Prob. 29PCh. 15 - A number of drugs are prepared by reactions in...Ch. 15 - The following conversion was carried out in two...Ch. 15 - Outline syntheses of (a)...Ch. 15 - (S)-(+)-Ibuprofen can be prepared by...Ch. 15 - Like other hydroborations, the reaction of alkynes...Ch. 15 - The sex attractant of the female silkworm has been...Ch. 15 - Prob. 36PCh. 15 - (a) Exaltolide, a musk substance, has been...Ch. 15 - Prob. 38PCh. 15 - Prob. 39PCh. 15 - Cyclobutadiene and...Ch. 15 - Cyclobutadiene and (Cyclobutadiene)tricarbonyliron...Ch. 15 - Cyclobutadiene and...Ch. 15 - Cyclobutadiene and...Ch. 15 - Cyclobutadiene and...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- give an example of linkage isomerism.arrow_forwardHeptacarbon octahydride is an effective gasoline additive. In the presence of oxygen gas (and a spark), it undergoes complete combustion. (a) Write the balanced equation for this reaction. Identify the oxidizing agent and reducing agent.arrow_forwardWhich of the following statements about the reaction H2(g)+O2(g)→H2O(l)H2(g)+O2(g)→H2O(l) is true? (i) This is an example of an acid–base reaction. (ii) O2O2 is oxidized in this reaction. (iii) H2H2 is reduced in this reaction. Which of the following statements about the reaction is true? (i) This is an example of an acid–base reaction. (ii) is oxidized in this reaction. (iii) is reduced in this reaction. iii only i only ii and iii ii only None of them are true.arrow_forward
- How many moles of (CH3)3N would need to be added to 459.2 mL of a 0.25 M solution of (CH3)3NHCL to produce a solution with a pH equal to 9.21?arrow_forward4) a) If 8.42 g of benzene is iodinated using ICl, what is the minimum mass of ICl that is required for the reaction? b) What mass of ICl corresponds to 1.35 equivalents?arrow_forwardA solution of tyrosine is made by dissolving 0.005 mole of tyrosine in 1.0 L of 0.1 M NaOH solution. Draw the structure of the principal chemical form of tyrosine found in this solution at equilibrium given the data below. NH2 pKa (CO2H) = 2.41 pKa (NH2) = 8.67 pka (OH) = 11.01 CHCH2 OH CO2Harrow_forward
- 18 19 20 21 22 23 24 Answer the questions in the table below about the shape of the methyl (CH3) anion. How many electron groups are around the central carbon atom? Note: one "electron group" means one lone pair, one single bond, one double bond, or one triple bond. 0 What phrase best describes the arrangement of these electron groups around the central carbon atom? (You may need to use the scrollbar to see all the choices.) ✓ (choose one) linear bent T-shaped trigonal planar trigonal pyramidal square planar square pyramidal tetrahedral sawhorse trigonal bipyramidal octahedral Continue Two Column N....pages Kindergarten O....docx Grey Black and B....pdf 14 431 10 25 nola Mir Grey Black and B....pdf 26 Ⓒ2022 McGraw Hill LLC. Allarrow_forwardWhat is the net ionic equation for the hydrolysis of NH4Cl?arrow_forwardThe zinc(II) ion behaves as a Lewis acid. It is found in an enzyme called carboxypeptidase A. This enzyme catalyzed the hydrolysis of C-terminal peptide bonds of proteins. The zinc ion will accept the lone pair of electrons on the oxygen in the peptide linkage. Explain why this Lewis acid behavior would make the carbon atom more susceptible to reaction.arrow_forward
- Write and balance the Chemical Equation:HC2H3O2 + CaCO3→ calcium acetate + H2O + carbon dioxidearrow_forwardWrite the chemical formula for ammonium oxalatearrow_forwardGive the chemical formula for (a) hydrocyanic acid,(b) nickel tetracarbonyl, (c) barium bicarbonate, (d) calciumacetylide, (e) potassium carbonatearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
 Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning


Macroscale and Microscale Organic Experiments
Chemistry
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Brooks Cole
Calorimetry Concept, Examples and Thermochemistry | How to Pass Chemistry; Author: Melissa Maribel;https://www.youtube.com/watch?v=nSh29lUGj00;License: Standard YouTube License, CC-BY