
Interpretation:
The structure of the compound of the formula
Concept introduction:
The structure of a compound can be determined from the IR and NMR spectra.
The IR spectrum of a molecule consists of several absorption peaks/bands. These bands, particularly those above about 1200
The
The
The formula of the compound, if known, can provide information about the unsaturation, number of double or triple bonds, and rings in the structure. This is known as the Index of Hydrogen Deficiency (IHD), and for a molecule containing only C, H, and O, it is calculated as
Using this information, the possible ways in which the fragments and functional groups are connected can be determined, leading to the probable structure of the molecule.
Answer to Problem 16.82P
The structure of the compound is
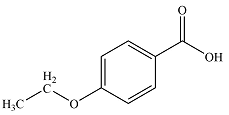
Explanation of Solution
The formula of the compound is
Considering the high value of IHD, it is likely that the compound contains a benzene ring. This will account for four, leaving one double bond in the substituent.
The presence of the two peaks at 6.9 and 7.0 ppm in the
The
The data from the spectrum can then be summarized as follows:
| δ ppm | No. of H’s | Splitting | Coupled H’s | Fragment |
| 1.4 | 3 | Triplet | 2 | 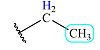 |
| 4.1 | 2 | Quartet | 3 | |
| 6.9 | 2 | Doublet | 1 | 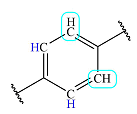 |
| 7.9 | 2 | Doublet | 1 | 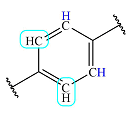 |
| 12.2 | 1 | Singlet | 0 | 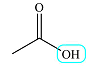 |
The three fragments – a di-substituted benzene ring
The presence of the carboxylic acid group is supported by the broad peak from about 2500
Thus, the molecular structure of the compound can be constructed by putting together the fragments so as to get para-disubstituted benzene. This structure shows seven structurally distinct carbons, matching the seven signals seen in the
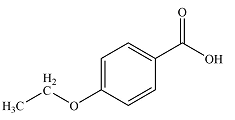
The structure of the compound was determined from the
Want to see more full solutions like this?
Chapter 16 Solutions
Organic Chemistry: Principles and Mechanisms (Second Edition)
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





