
a)
Interpretation:
The structural formula of 2, 3- dimethylheptane should be stated.
Concept Introduction:
The systematic structure determination of organic compound is given by IUPAC. The name of the organic compound is determined from the structure that is by analyzing the position of groups substituted on it.
a)
Answer to Problem 63A
The structural formula of the compound 2, 3- dimethylheptane is:
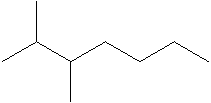
Explanation of Solution
In the structure; the parent atom contains seven carbon atom.so, the word root is heptane.
At 2 and 3 position there are two methyl groups which is named as dimethyl.
Therefore; the structure of the given molecule is

(b)
Interpretation:
The structural formula of 2, 2-dimethyl-3-chloro-1-octanol should be stated.
Concept Introduction:
The systematic structure determination of organic compound is given by IUPAC. The name of the organic compound is determined from the structure that is by analyzing the position of groups substituted on it.
(b)
Answer to Problem 63A
The structural formula of the compound 2, 2-dimethyl-3-chloro-1-octanol is
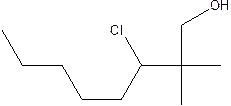
Explanation of Solution
In the structure; the parent chain contains eight atoms so; the word root is octane.
At position 2 there are 2 methyl groups are present which is named as dimethyl, at 3rd position chlorine is present which is named as chloroand at position 1, the OH group is present which is names as ‘ol’
Therefore, the structure of the given molecule is
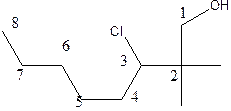
C)
Interpretation:
The structural formula of 2-chloro-1-hexene should be stated.
Concept Introduction:
The systematic structure determination of organic compound is given by IUPAC. The name of the organic compound is determined from the structure that is by analyzing the position of groups substituted on it.
C)
Answer to Problem 63A
The structural formula ofthe compound 2-chloro-1-hexene is
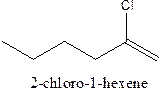
Explanation of Solution
In the structure; the parent chain contains the six carbon atoms and the word root is hexane.
At position 1, there is
Therefore; the structure of the given molecule is
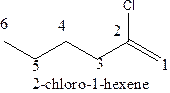
d)
Interpretation:
The structural formula of 1- chloro-2-hexene should be stated.
Concept Introduction:
The systematic structure determination of organic compound is given by IUPAC. The name of the organic compound is determined from the structure that is by analyzing the position of groups substituted on it.
d)
Answer to Problem 63A
The structural formula ofthe compound 1- chloro-2-hexene is
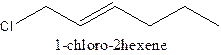
Explanation of Solution
In the structure; the parent chain contains the six carbon atoms and the word root is hexane.
At position 1, there is chlorine group which is named as chloroand at position 2, alkene group is present.which is names as ‘ene’.
Therefore, the structure of the given molecule is
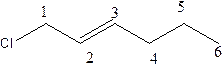
e)
Interpretation:
The structural formula of 2- methylphenol should be stated.
Concept Introduction:
The systematic structure determination of organic compound is given by IUPAC. The name of the organic compound is determined from the structure that is by analyzing the position of groups substituted on it.
e)
Answer to Problem 63A
The structural formula of the compound 2- methylphenol is
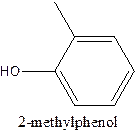
Explanation of Solution
In the structure, the parent ring contains six carbon atom which is named as benzene ring. At position 1, the methyl group present and at 2nd position there is OH group present which is named as ‘ol’.
Therefore; the structure of the given molecule is
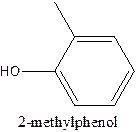
Chapter 20 Solutions
World of Chemistry
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





