
Organic Chemistry - Standalone book
10th Edition
ISBN: 9780073511214
Author: Francis A Carey Dr., Robert M. Giuliano
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 27, Problem 22P
(a) The two most acidic hydrogens of uracil have
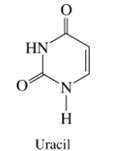
(b) The
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Given the structure of ascorbic acid (vitamin C):(a) Is ascorbic acid a carboxylic acid?(b) Compare the acid strength of ascorbic acid (pKa = 4.71) with acetic acid.(c) Predict which proton in ascorbic acid is the most acidic.(d) Draw the form of ascorbic acid that is present in the body (aqueous solution, pH = 7.4)
Given that C6H11COOH has a pKa = 4.8 and C6H11N+H3 has a pKa = 10.7,
(a) What pH would you make the water layer to cause the carboxylic acid to dissolve in the water layer and the amine to dissolve in the ether layer? (b) What pH would you make the water layer to cause the carboxylic acid to dissolve in the ether layer and the amine to dissolve in the water layer?
(a) Predict the product of the reaction of KOH with 1-amino propane.
(b) Predict the product of a deprotonated ethanol (an “ethanolate anion", O-CH2-CH3)
with phenol (hydroxybenzene).
(c) Predict the product of propanoic acid with deprotonated ethanol (an “ethanolate
anion", O-CH2-CH3).
Chapter 27 Solutions
Organic Chemistry - Standalone book
Ch. 27.1 - Problem 27.1 Write a structural formula for the...Ch. 27.1 - Prob. 2PCh. 27.1 - Prob. 3PCh. 27.2 - Prob. 4PCh. 27.3 - Prob. 5PCh. 27.3 - Prob. 6PCh. 27.5 - Prob. 7PCh. 27.5 - Prob. 8PCh. 27.5 - Prob. 9PCh. 27.6 - Prob. 10P
Ch. 27.7 - Prob. 11PCh. 27.9 - Prob. 12PCh. 27.12 - 27.13 Modify Figure 27.12 so that it corresponds...Ch. 27.13 - Prob. 14PCh. 27 - Prob. 15PCh. 27 - Prob. 16PCh. 27 - Prob. 17PCh. 27 - Nebularine is a toxic nucleoside isolated from a...Ch. 27 - Prob. 19PCh. 27 - The 5-nucleotide of inosine, inosinic acid...Ch. 27 - Prob. 21PCh. 27 - (a) The two most acidic hydrogens of uracil have...Ch. 27 - The phosphorylation of -D-glucopyranose by ATP...Ch. 27 - When 6-chloropurine is heated with aqueous sodium...Ch. 27 - Prob. 25PCh. 27 - Prob. 26PCh. 27 - Prob. 27PCh. 27 - Prob. 28PCh. 27 - Oligonucleotide Synthesis In Section 27.6 we noted...Ch. 27 - Oligonucleotide Synthesis In Section 27.6 we noted...Ch. 27 - Oligonucleotide Synthesis In Section 27.6 we noted...Ch. 27 - Oligonucleotide Synthesis In Section 27.6 we noted...Ch. 27 - Oligonucleotide Synthesis In Section 27.6 we noted...Ch. 27 - Oligonucleotide Synthesis In Section 27.6 we noted...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Complete the following equation for the dissociation of butyric acid in water, so as to illustrate unambiguously that butyric acid is a weak acid: CH3CH2CH2COOH(aq)+ H20(1)arrow_forwardUsing the data in Appendix C, determine which of the following bases is strong enough to deprotonate acetonitrile (CH3CN), so that equilibrium favors the products: (a) NaH; (b) Na2CO3; (c) NaOH; (d) NaNH2; (e) NaHCO3.arrow_forwardProvide an explanation without using the pka values : Why is phenol stronger acid than butanoic acid?arrow_forward
- The Ka1 of ascorbic acid is 7.94 x 10-5. Would you expect ascorbic acid dissolved in blood plasma (pH 7.35–7.45) to exist primarily as ascorbic acid or as ascorbate anion? Explain.arrow_forwardA dibasic organic acid has a neutralization equivalent of 45+1. Deduce the structure of this organic acidarrow_forward18-48 4-Aminobenzoic acid is prepared from benzoic acid by the following two steps. Show reagents and experimental conditions to bring about each step.arrow_forward
- A chemically modified guanidino group is present in cimetidine (Tagamet), a widely prescribed drug for the control of gastric acidity and peptic ulcers. Cimetidine reduces gastric acid secretion by inhibiting the interaction of histamine with gastric H2 receptors. In the development of this drug, a cyano group was added to the substituted guanidino group to alter its basicity. Do you expect this modified guanidino group to be more basic or less basic than the guanidino group of arginine? Explain.arrow_forwardThe pKa of the conjugate acid of guanidine is 13.6, making it one of thestrongest neutral organic bases. Offer an explanation.arrow_forward(a) Explain how NaBH, in CH;OH can reduce hemiacetal A to 1,4-butanediol (HOCH,CH,CH,CH,OH). (b) What product is formed when A is treated with Ph;P=CHCH,CH(CH),? (c) The drug isotretinoin is formed by reaction of X and Y. What is the structure of isotretinoin? Although isotretinoin (trade name Accutane or Roaccutane) is used for the treatment of severe acne, it is dispensed under strict controls because it also causes birth defects. PPha NaOCH,CH3 HO- isotretinoin HO A Br X Yarrow_forward
- (a) Draw the structures of ammonia and urea.(b) Write the reaction showing ammonia acting as a base in water.(c) Write a reaction showing the formation of carbamoyl phosphate from ammonia, carbon dioxide, and any other reactants.(d) Draw the structures of ornithine and lysine. Explain how they are different. (e) Draw the structure of the compound formed when ornithine reacts with carbamoyl phosphate. (f) Write the transamination reaction of alanine with alpha-ketoglutarate. Name the products that are formed.arrow_forward18:3c∆9,12,15 all cis-9,12,15-octadecatrienoic, linolenic acid cis-9,12-octadecadienoic, linoleic acid cis-9,12-octadecadienoic, linolenic acid all cis-9,12,15-octadecatrienoic, arachidonic acidarrow_forwardDraw a flow sheet to show how you would separate the components of a mixture containing an acid substance, toluic acid, a basic substance, p-bromoaniline, and anthracene, a neutral substance.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Brooks Cole

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning
Enzymes - Effect of cofactors on enzyme; Author: Tutorials Point (India) Ltd;https://www.youtube.com/watch?v=AkAbIwxyUs4;License: Standard YouTube License, CC-BY
Enzyme Catalysis Part-I; Author: NPTEL-NOC IITM;https://www.youtube.com/watch?v=aZE740JWZuQ;License: Standard Youtube License