
Study Guide for Campbell Biology
11th Edition
ISBN: 9780134443775
Author: Lisa A. Urry, Michael L. Cain, Steven A. Wasserman, Peter V. Minorsky, Jane B. Reece, Martha R. Taylor, Michael A. Pollock
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 5, Problem 3IQ
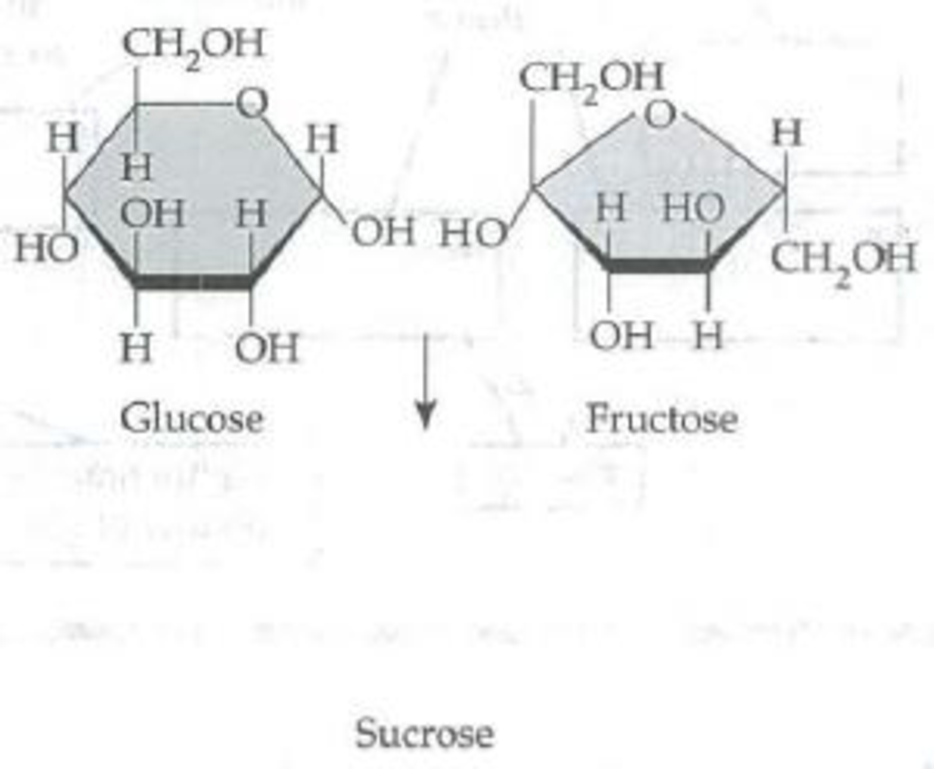
Number the carbons in the following glucose and fructose molecules (each unlabeled corner of the ring represents a carbon. In glucose, carbon 1 is to the right of the O in the ring; in fructose, carbon 1 extends up from the ring on the left side.) Circle the atoms that will be removed by a dehydration reaction. Then draw the resulting sucrose molecule with its 1–2 glycosidic linkage.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
In the structure in the picture, the monosaccharide units (alpha-D-galactopyranosyl and beta-D-allopyranose) are linked via alpha-1->3 glycosidic bond. Then, the formed disaccharide units are linked via beta-1->4 glycosidic bond. Then, an Oligosaccharide is formed which has 10 monosaccharide units, meaning that it contains 5 disaccharide units.
Question:
Is this oligosaccharide a good substrate for glycolysis? Provide two reasons for your answer.
1) Please list all glycosidic linkages between each monosaccharide units. For example, α(1→4)2) Please discuss whether these oligo/polysaccharides would be reducing or non-reducing sugar. Remember to state your reasoning in complete sentence.
Although the first two carbons of fructose and glucose are identical in structure to DHAP and GADP (from glycolysis), DHAP and GADP equilibriate on their in solution to favor the ketone over the aldehyde, while fructose and glucose do not. Why?
a)The larger size of the molecule sterically hinders the isomerization
b)The larger sugars have more OH groups which hydrogen bond and disrupt isomerization
c)The larger sugars cyclize, and there is no carbonyl to isomerize in the cyclic form
d)The larger sugars cyclize, and in the cyclic form the hydrogen bonding is very strong
e)The larger sugars are less soluble in water than the smaller sugars
Chapter 5 Solutions
Study Guide for Campbell Biology
Ch. 5 - Monomers are linked into polymers by ________...Ch. 5 - You can recognize a monosaccharide by its multiple...Ch. 5 - Number the carbons in the following glucose and...Ch. 5 - Prob. 4IQCh. 5 - Fill in this concept map to help you organize your...Ch. 5 - a. Draw the amino acids alanine (R group: CH3) and...Ch. 5 - In the following diagram of a portion of a...Ch. 5 - Now that you have gained experience with concept...Ch. 5 - a. Label the three parts of this nucleotide....Ch. 5 - Take the time to create a concept map that...
Ch. 5 - Prob. 1SYKCh. 5 - Prob. 2SYKCh. 5 - glycogen A. carbohydrate B. lipid C. protein D....Ch. 5 - cholesterol A. carbohydrate B. lipid C. protein D....Ch. 5 - RNA A. carbohydrate B. lipid C. protein D. nucleic...Ch. 5 - collagen A. carbohydrate B. lipid C. protein D....Ch. 5 - hemoglobin A. carbohydrate B. lipid C. protein D....Ch. 5 - A. carbohydrate B. lipid C. protein D. nucleic...Ch. 5 - Prob. 7TYKMCh. 5 - enzyme A. carbohydrate B. lipid C. protein D....Ch. 5 - cellulose A. carbohydrate B. lipid C. protein D....Ch. 5 - Chitin A. carbohydrate B. lipid C. protein D....Ch. 5 - Polymerization (the formation of polymers) is a...Ch. 5 - Which of the following statements is not true of a...Ch. 5 - Prob. 3TYKCh. 5 - Prob. 4TYKCh. 5 - Prob. 5TYKCh. 5 - Prob. 6TYKCh. 5 - A fatty acid that has the formula C16H32O2 is a....Ch. 5 - Prob. 8TYKCh. 5 - Prob. 9TYKCh. 5 - Prob. 10TYKCh. 5 - Which of the following molecules provides the most...Ch. 5 - Prob. 12TYKCh. 5 - What happens when a protein denatures? a. Its...Ch. 5 - The helix of proteins is a. part of a proteins...Ch. 5 - What is the best description of the following...Ch. 5 - Prob. 16TYKCh. 5 - Prob. 17TYKCh. 5 - Which of the following is true of the subunits of...Ch. 5 - Prob. 19TYKCh. 5 - If the nucleotide sequence of one strand of a DNA...Ch. 5 - How are nucleotide monomers connected to form a...Ch. 5 - Prob. 22TYK
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Similar questions
- An oligosaccharide is a repeating unit of a-D-galactopyranosyl-(a-1 >3)-allopyranoside. Each disaccharide unit is linked via B-1 --->4 glycosidic bond. The oligosaccharide has 10 monosaccharide residues. Required: Is this oligosaccharide a good substrate for glycolysis? Why or why not? Provide two reasons and discuss corn prehensively.arrow_forwardAnswer the following questions regarding the trisaccharide composed of a glucose, mannose and galactose a) What is the configuration on the anomeric carbon for the each of the sugars b) Which sugar end(s) are reducing? c) What is the numbering on the linkage connecting the glucose to the mannose? d) What is the numbering on the linkage connecting the mannose to the galactose? OH CH,OH Galactose но. но, CH,OH но но. он Glucose но. он Mannosearrow_forwardAn amylose chain is 5000 glucose units long. At how many places must it be cleaved to reduce the average length to 2500 units? To 1000 units? To 200 units? What percentage of the glycosidic links are hydrolyzed in each case? (Even partial hydrolysis can drastically alter the physical properties of polysaccharides and thus affect their structural role in organisms.)arrow_forward
- Assume you could make a trisaccharide by covalently joining xylose (C5H10O5) , xylose (C5H10O5) and fructose (C6H12O6). So 2x xylose, 1x fructose. What would be the chemical formula of this trisaccharide assuming regular dehydration synthesis as discussed in class? You do not need to worry about which carbons are being used for forming these glycosidic linkages.arrow_forwarddraw disaccharide as Haworth projection: (shown in image) -is this drawing a reducing sugar? Briefly explainarrow_forwarddraw the following amino acid chains and give the single and three letter abbreviations for cysteinearrow_forward
- Based on the structure of sucrose below, is it classified as a reducing sugar? ОН 6CH OH 5 5 ОН 3 6CH OH a-Glucose 2 ОН 0. ОН HO 3 1 ОН ОН 2 CH₂OH 1 B-Fructose ОН CH₂OH ОН CH₂OH ОН OH НО a, B (1-2) linkage CH₂OH Sucrose + H2Oarrow_forwardName the three digestible disaccharides we talked about. What monosaccharides are they each made of and tell whether they are connected by an alpha 1-4 glycosidic linkage, a 1-4 beta glycosidic linkage or a 1-5 beta glycosidic linkage.arrow_forwardConsider the following statements: (1) The term sugar is a general designation for both monosaccharides and disaccharides. (2) The "penultimate carbon" in a monosaccharide is used to determine D- or L-configuration. (3) Sucrose is a reducing sugar and lactose is a nonreducing sugar. O Two of the three statements are true. All three statements are true. O Only one of the statements is true. None of the statements are true.arrow_forward
- Draw the structure of: The polysaccharide in (b) is dextran, a component of dental plaque. Here b: (b) a polysaccharide formed by joining D-glucose units in 1→6-α-glycosidic linkagesarrow_forwardWhen glucose is reduced, only one alditol is produced. When fructose undergoes the same reaction, however, two diasteriometric sugars are produced. Draw their structures.arrow_forwardWhy is lactose a reducing sugar while sucrose is a non-reducing sugar? Explain clearly. Please support the answer with an illustration.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON
Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education,
Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education, Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company
Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co.
Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co. Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education
Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education

Human Anatomy & Physiology (11th Edition)
Biology
ISBN:9780134580999
Author:Elaine N. Marieb, Katja N. Hoehn
Publisher:PEARSON

Biology 2e
Biology
ISBN:9781947172517
Author:Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:OpenStax

Anatomy & Physiology
Biology
ISBN:9781259398629
Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa Stouter
Publisher:Mcgraw Hill Education,

Molecular Biology of the Cell (Sixth Edition)
Biology
ISBN:9780815344322
Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter Walter
Publisher:W. W. Norton & Company

Laboratory Manual For Human Anatomy & Physiology
Biology
ISBN:9781260159363
Author:Martin, Terry R., Prentice-craver, Cynthia
Publisher:McGraw-Hill Publishing Co.

Inquiry Into Life (16th Edition)
Biology
ISBN:9781260231700
Author:Sylvia S. Mader, Michael Windelspecht
Publisher:McGraw Hill Education
Macromolecules | Classes and Functions; Author: 2 Minute Classroom;https://www.youtube.com/watch?v=V5hhrDFo8Vk;License: Standard youtube license