
Organic Chemistry As a Second Language: Second Semester Topics
4th Edition
ISBN: 9781119110651
Author: David R. Klein
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Question
Chapter 5.3, Problem 5.17P
Interpretation Introduction
Interpretation:
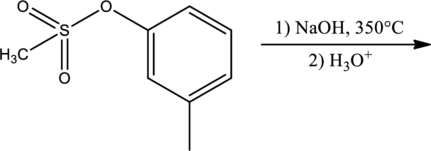
Products for the given reaction has to be predicted.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Predict the products of this organic reaction:
CH3 CH₂
||
No reaction
CH3
|
O−CH–CH2–CH3
+ KOH
A
Specifically, in the drawing area below draw the structure of the product, or products, of this reaction. (If there's more than one product, draw them in any
arrangement you like, so long as they aren't touching.)
If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area.
Click anywhere to draw the first
atom of your structure.
X
Ś
Ć
Br
CH3OH
+
Br-Br
H3CO
The mechanism proceeds through a first cationic intermediate, intermediate 1. Nucleophilic attack leads to intermediate 2, which goes on to form the
final product. In cases that involve a negatively charged nucleophile, the attack of the nucleophile leads directly to the product.
+Br
+ CH3OH
Br
Intermediate 1
Intermediate 2
(product)
In a similar fashion, draw intermediate 1 and intermediate 2 (final product) for the following reaction.
OH
+ Br2
+ HBr
Br
racemic mixture
• Pay attention to the reactants, they may differ from the examples. In some reactions, one part of the molecule acts as the nucleophile.
• You do not have to consider stereochemistry.
• Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. Separate intermediate 1 and
intermediate 2 using the → symbol from the dropdown menu.
Alcohols are acidic in nature. Therefore, a strong base can
abstract the acidic hydrogen atom of the alcohol in a process
known as deprotonation. The alcohol forms an alkoxide ion by
losing the proton attached to the oxygen atom of the hydroxyl (
-OH) group. The alkoxide formed can act as a base or a
nucleophile depending on the substrate and reaction conditions.
However, not all bases can abstract the acidic proton of alcohols
and not all alcohols easily lose the proton. Deprotonation
depends on the strength of the base and the acidity of the
alcohol. Strong bases, such as NaNH2, can easily abstract a
proton from almost all alcohols. Likewise, more acidic alcohols
lose a proton more easily.
Determine which of the following reactions would undergo deprotonation based on the strength of the base and the acidity of the alcohol.
Check all that apply.
► View Available Hint(s)
CH3CH,OH + NH3 →CH,CH,O-NH
CH3
CH3
H3C-C-H+NH3 → H3 C-C-H
OH
O-NH
CH3CH2OH + NaNH, → CH3CH,O-Na* + NH3
CHC12
Cl₂…
Chapter 5 Solutions
Organic Chemistry As a Second Language: Second Semester Topics
Ch. 5.1 - Prob. 5.2PCh. 5.1 - Prob. 5.3PCh. 5.1 - Prob. 5.4PCh. 5.1 - Prob. 5.5PCh. 5.1 - Prob. 5.6PCh. 5.1 - Prob. 5.7PCh. 5.2 - Propose a mechanism for each of the following...Ch. 5.2 - Propose a mechanism for each of the following...Ch. 5.2 - Propose a mechanism for each of the following...Ch. 5.3 - Prob. 5.13P
Ch. 5.3 - Prob. 5.14PCh. 5.3 - Prob. 5.15PCh. 5.3 - Prob. 5.16PCh. 5.3 - Prob. 5.17PCh. 5.3 - Prob. 5.18PCh. 5.4 - Propose a mechanism for each of the following...Ch. 5.4 - Propose a mechanism for each of the following...Ch. 5.4 - Propose a mechanism for each of the following...Ch. 5.4 - Propose a mechanism for each of the following...
Knowledge Booster
Similar questions
- Acyl transfer (nucleophilic substitution at carbonyl) reactions proceed in two stages via a "tetrahedral intermediate." Draw the tetrahedral intermediate as it is first formed in the following reaction. CH3OH H3C CI • • • • You do not have to consider stereochemistry. Include all valence lone pairs in your answer. Do not include counter-ions, e.g., Na+, I", in your answer. In cases where there is more than one answer, just draw one.arrow_forwardPredict the products of this organic reaction: CH3 CH₂ || No reaction CH3 -CH-CH₂-CH3 + NaOH A Specifically, in the drawing area below draw the structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. ? Click anywhere to draw the first atom of your structure. X Śarrow_forwardMECHANISM ACTIVITY Base-Induced Ester Hydrolysis (Saponification) NOC XT Step 1: Nucleophilic addition of hydroxide ion to the ester carbonyl group Ahrs MPR R16 H3C. R Part A (1 of 2) Draw the curved arrows for Step 1 of this mechanism. Arrow-pushing Instructions CH3 H-O: ÖH OR' :Ö H-O: O: H H H H 1x xxx CH3 CH3 Step 1 H3C. CH3 заarrow_forward
- Compare the two reactions shown below. excess LICH,CH3 excess LICH,CH3 Both electrophiles, an ester and a ketone, are reacted with an excess amount of LICH2CH2. Which reaction will result in the formation of a product with the molecular formula C9H200 and why? O A: Only the reaction with the ester. LICH2CH3 results in an addition mechanism with both starting materials. However, the product from the ketone reaction contains fewer than 9 carbons. O B. Only the reaction with the ketone. LICH2CH3 results in an addition mechanism with both starting materials. However, the product from the ester reaction contains two oxygen atoms. O C. Both reactions. LICH2CH3 results in an addition mechanism with the ketone. However, the ester goes through a SNAC mechanism because it contains a leaving group, which allows two equivalents of LICH2CH3 to attack. O D. Neither reaction. LICH2CH3 results in a SNAC mechanism with both starting materials. Both products contain more than 9 carbons.arrow_forwardDraw the simplest mechanism possible for the reaction below. You may need to re-draw structures to show bond lines or lone pairs. Note to advanced students: There may be more than one resonance structure for one of your products. Make sure the mechanism you draw creates the resonance structure that's shown. + но + H₂Oarrow_forward1a) Provide the mechanism of the reaction below, catalyzed by triethylamine.The reaction should take you three mechanistic steps. Referring to the C=O group that already exists in the starting molecule: b) If this ketone were not present (for example, if it were just an H there instead), would the first step be faster or slower? Briefly explain. c) If this ketone were not present (for example, if it were just an H there instead), would the second step be faster or slower? Briefly explain. d) If 9.21 g of the starting reactant is used and 0.27 g of triethylamine are used, approximately how many reactions will each molecule of triethylamine catalyze? (hint: balance the equation)arrow_forward
- Please answer the two items. The following compounds are major products of an elimination reaction with an alkyl halide. Determine the structure of the alkyl halide. Write the overall reaction mechanism. Tip: Remember your Markovnikov’s rule. Follow the progress of the reaction and look at the stability of the intermediate products and transition states to determine the minor and major products.arrow_forwardThe dehydration of compound A produces many different products. One of the products formed is compound G. Can you figure out the mechanism? The mechanism proceeds through five intermediates (compounds B-F). Sort the intermediates according to the reaction mechanism which includes a methyl shift, a hydride shift and a resonance structure. Note: treat resonance structures as individual steps. Compound III Compound IV Compound II Compound I Compound V Your answer compound A OH compound G intermediates IIarrow_forwardFor each of the molecules below, identify whether the alkene has E or Z configuration. Molecule F H H • E or Z? OE Z ΟΕ ΟΖ Molecule F LL CN ОН NH₂ E or Z? OZ ΟΕ OZarrow_forward
- For the following reaction explain why the unexpected products form, while the two theoretical products below are not formed.arrow_forward3) Fill in the red box(es) with the missing reactant(s), reagent(s), product(s), solvent, and/or conditions and write a reasonable mechanism for the reaction. Not every box needs to be used and not every box necessarily corresponds to only a single species. If no reaction occurs OR no reagents exist to perform the reaction state, "NOT POSSIBLE" AND explain why. os 1) NaOH 2) H₂O*arrow_forwardConstruct a three-step synthesis of 1-bromopropane from propane by dragging the appropriate formulas into the bins. Note that each bin willl hold only one item, and not all of the given reagents or structures will be used. Reactant Reagent 1 Step 1 Product Reagent 2 Step 2 Product Reagent 3 Final Product (1-bromopropane) (propane) Br2 Br2 (CH)3CO K HBr ROOR HBr NBS ROOR HaC Нас Нас, Нас Нас НаС. hv CH2 CH2 CH Нс CH-Br CH-Br CH Нас Нас, нC Нас НаС Br Br Brarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning