
EBK ORGANIC CHEMISTRY-PRINT COMPANION (
4th Edition
ISBN: 9781119776741
Author: Klein
Publisher: WILEY CONS
expand_more
expand_more
format_list_bulleted
Question
Chapter 6, Problem 49ASP
Interpretation Introduction
Interpretation: The type of the reaction step is to be interpreted in the given options.
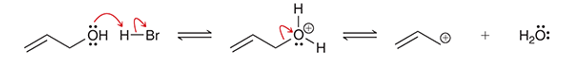
Concept introduction: In a
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Provide an appropriate alkyne starting material A and intermediate product B. Omit byproducts. The number of carbon atoms in
the starting material should be the same as in the final product.
1. R2BH,
THE
Alkyne
Intermediate
Starting
Material
2. H2O2,
NaOH, H2O
A
Draw intermediate B.
Draw alkyne starting material A.
Rings
More
Erase
Select
Draw
Rings
More
Erase
Select
Draw
H
H
What explains why many aldehydes and ketones can undergo self- condensation reactions in basic conditions?
A. The alpha carbon can lose a proton and act like a nucleophile and the carbonyl carbon a an electrophile
B. The alpha carbon can gain a proton and act like an electrophile and the carbonyl carbon is a nucleophile
C. The oxygen of the carbonyl group can attack the carbon of the carbonyl group
D. Only esters can undergo self-condensation reactions
Select all that belongs to "Electrophile"
accepts an electron pair to form a bond
donates an electron pair to form a bond
Weak Lewis Acid
Electron rich
Electron poor
Substitutes leaving group in Sn1 and Sn2 reactions
Partially positive
Strong Lewis Acid
MacBook Air
F6
F7
F8
F9
F10
F4
F5
%
&
5
6
7
8
Chapter 6 Solutions
EBK ORGANIC CHEMISTRY-PRINT COMPANION (
Ch. 6.1 - Prob. 1LTSCh. 6.1 - Prob. 1PTSCh. 6.1 - Prob. 2ATSCh. 6.2 - Prob. 3CCCh. 6.3 - Prob. 4CCCh. 6.3 - Prob. 5CCCh. 6.4 - Prob. 6CCCh. 6.6 - Prob. 7CCCh. 6.7 - Prob. 2LTSCh. 6.7 - Prob. 8PTS
Ch. 6.7 - Prob. 9PTSCh. 6.7 - Prob. 10ATSCh. 6.8 - Prob. 3LTSCh. 6.8 - Prob. 11PTSCh. 6.8 - Prob. 12ATSCh. 6.9 - Prob. 4LTSCh. 6.9 - Prob. 13PTSCh. 6.9 - Prob. 14ATSCh. 6.10 - Prob. 5LTSCh. 6.10 - Prob. 15PTSCh. 6.10 - Prob. 16ATSCh. 6.11 - Prob. 6LTSCh. 6.11 - Prob. 17PTSCh. 6.11 - Prob. 18ATSCh. 6 - Prob. 19PPCh. 6 - Prob. 20PPCh. 6 - Prob. 21PPCh. 6 - Prob. 22PPCh. 6 - Prob. 24PPCh. 6 - Prob. 25PPCh. 6 - Prob. 26PPCh. 6 - Prob. 27PPCh. 6 - Prob. 28PPCh. 6 - Prob. 29PPCh. 6 - Prob. 30PPCh. 6 - Prob. 31PPCh. 6 - Prob. 32PPCh. 6 - Prob. 33PPCh. 6 - Prob. 34PPCh. 6 - Prob. 35PPCh. 6 - Prob. 36PPCh. 6 - Prob. 37PPCh. 6 - Prob. 38PPCh. 6 - Prob. 39PPCh. 6 - Prob. 40PPCh. 6 - Prob. 41PPCh. 6 - Prob. 43ASPCh. 6 - Prob. 44ASPCh. 6 - Prob. 45ASPCh. 6 - Prob. 46ASPCh. 6 - Prob. 47ASPCh. 6 - Prob. 48ASPCh. 6 - Prob. 49ASPCh. 6 - Prob. 50IPCh. 6 - Prob. 51IPCh. 6 - Prob. 52IPCh. 6 - Prob. 53IPCh. 6 - Prob. 54IPCh. 6 - Prob. 55IPCh. 6 - Prob. 56IPCh. 6 - Prob. 57IPCh. 6 - Prob. 58IPCh. 6 - Prob. 59IPCh. 6 - Prob. 60IPCh. 6 - Prob. 61IPCh. 6 - Prob. 62CPCh. 6 - Prob. 64CP
Knowledge Booster
Similar questions
- MCQ 19: Ion which is more effective nucleophile than water is A. negatively charged hydroxide B. carbocation C. anion D. hydroxyl ionarrow_forward1. Complete the following reactions. a. b. C. N HIN 1. LiAlH4 2. H₂O* 1. NaAlH4 2. H₂O* 1. LiAlH4 2. H₂O*arrow_forwardIdentify the role/function of the indicated molecule in the reaction? 1. LIAIH, ether 2. Но NaN3 Br -NH2 a. Nucleophile b. Electrophile c. Base O d. Acidarrow_forward
- Alkynes exhibit acidic properties because of the differences in the electronegativity of sp- hybridized carbons. Which of the following statements best explains the acidic property of acetylene? A. The electronegativity of sp hybridized carbon decreases bond dipole between C and H. B. Hydrogen atom becomes ionized due to sp-s repulsion. C. The sp-sp orbital overlap attracts the electron cloud between C and H. D. The pi bonds in the triple bond promote ionization of carbon into carbocationsarrow_forwardA terminal alkyne is more acidic than an alkene and alkane because after deprotonation the lone pair will reside in an: A. p orbital B. sp orbital C. sp? orbital D. sp3 orbital Aarrow_forwardWhat are the steps and reagents used?arrow_forward
- Unanswered A Su Pre-lab question 3 Homework Unanswered The borohydride reduction of a ketone involves a nucleophilic attack on the electrophilic ketone carbon. The attacking nucleophile is? Select an answer and submit. For keyboard navigation, use the up/down arrow keys to select an answer. The boron atom of sodium borohydride. The hydrogen atom (acting as a hydride) of the sodium borohydride. The hydrogen atom (acting as a proton) of the sodium borohydride. The sodium atom of sodium borohydride. Show MacBook Pro P.arrow_forward9. Which of the compounds listed below is more acidic than 1-butanol? a) Diethylmalonate b) 2-Butanone c) Ethyl pentanoate d) All of these choices. e) Two of these choices. 11. Circle the Roman numeral corresponding to the most acidic hydrogen in the molecule below. III ve Î IV I II 10. Which of the compounds listed below is more acidic than 2- pentanol? a) Ethyl 3-oxopentanoate b) 2-Pentanone c) Pentanal d) All of these choices. e) Two of these choices. 12. Which of the following represent keto-enol tautomers? a) || CH3CCH₂CH3 and b) OH I CH₂=CCH₂CH3 c) HOCH₂CCH=CH₂ and and OH I CH3C=CHCH3 i CH3CCH₂CH3 CH3CCH₂CH3arrow_forwardWhat is required to complete the following reaction? H3C H 1) CH3CH₂MgBr, ether 2) H3O+ 1) LDA, THF 2) CH3CH₂Br 1) NaOH 2) CH3CH₂MgBr, ether 1) CH3CH₂Br 2) H3O+ H3C H₂ Harrow_forward
- Draw the products of the reaction and explan the process or mechanism. 1. H2SO4 НО H. loss of H2O 2. CH,CH2 H Acid catalyst C=C H Harrow_forwardWhy is it important that we are very careful in how much acid is added to neutralize the reaction? (Only pick one answer) If the solution becomes very acid it can be harmful to the skin Too much acid will increase vanillyl alcohols water solubility and inhibit crystal formation Too much acid will decrease vanillyl alcohols water solubility and inhibit crystal formation Excess acid will deprotonate the vanillyl alcohol and inhibit crystal formationarrow_forwardNucleophilic carbon atoms are often important in the formation of carbon–carbon bonds. Carbon atoms are not good nucleophiles if they do not possess a lone pair of electrons.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY