
Rearrange the Clausius-Clapeyron equation, equation 6.14 in terms of the pressure
Interpretation:
The vapor pressures of
Concept introduction:
The Clausius-Clapeyron equation can be obtained from the rearrangement and integration of Clapeyron equation. The Clausius-Clapeyron equation is generally used for gas-phase equilibria, to predict the equilibrium temperatures and pressures and also for the determination of enthalpy for phase transition.
Answer to Problem 6.70E
The plot between vapor pressure and temperature for
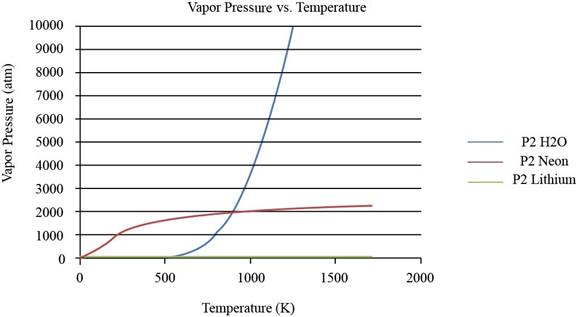
Figure 1
From the plot, the common observation is that for the given substances the increase in vapor pressure is slow until the normal boiling point.
Explanation of Solution
The Clausius-Clapeyron equation 6.14 is,
Rearrange the given equation for the partial pressure
Given boiling point of
Calculation of partial pressure
Calculation of partial pressure
Calculation of partial pressure
The plot between vapor pressure and temperature for
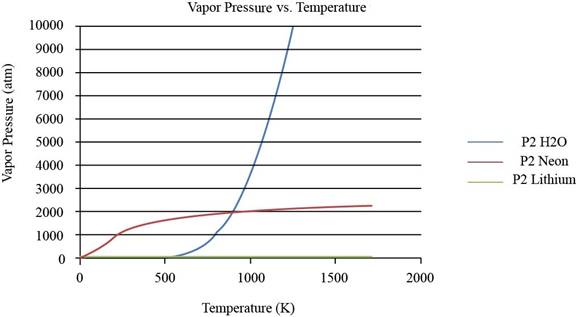
Figure 1
To observe the change in the plot of lithium, the values of vapor pressure is considered till
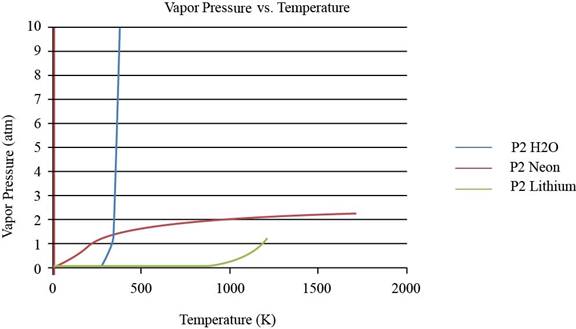
Figure 2
From the plot, the common observation is that for the given substances the increase in vapor pressure is slow until the normal boiling point. After the normal boiling point, the increase in vapor pressure is exponential. As the temperature increases from lower normal boiling point to higher values, the exponent value changes from negative to positive.
From the plot, the common observation is that for the given substances, the increase in vapor pressure is slow until the normal boiling point.
Want to see more full solutions like this?
Chapter 6 Solutions
Physical Chemistry
- Arrange the following substances in order of increasing strength of crystal forces: CO2, KCl, H2O, N2, CaO.arrow_forwardThe normal boiling point of SO2 is 263.1 K and that of NH3 is 239.7 K. At −40 °C, would you predict that ammonia has a vapor pressure greater than, less than, or equal to that of sulfur dioxide? Explain.arrow_forwardCalculate the heat (in kJ) required to transform 67.70 g of nitric acid from a solid at a temperature of -42 °C to a gas at 111 °C. Report your answer to one decimal place. Data: Molar mass of nitric acid, HNO 3 = 63.013 g/mol Melting point = -42 °C Boiling point = 83°C. Enthalpy of fusion = 10.5 kJ/mol Enthalpy of vaporization = 39.1 kJ/mol. Molar heat capacity of the liquid phase = 109.9 J/mol • K Molar heat capacity of the gas phase= 53.4 J/mol • K.arrow_forward
- A 7.40 g sample of solid Ni(CN), ·4H,0 was heated such that the water turned to steam and was driven off. Assuming ideal behavior, what volume would that steam occupy at 1.00 bar and 100 °C? It may be useful to consult the periodic table. volume: Larrow_forward10.) Diethyl ether is a volatile, highly flammable organic liquid that is used mainly as a solvent. The vapor pressure of diethyl ether is 401 mmHg at 18 degrees C. Calculate its vapor pressure at 52 degrees C.arrow_forwardHow much heat is required to convert 422 g of liquid H2O at 23.5 °C into steam at 150 °C?arrow_forward
- The melting point of copper metal is 1083 °C. The vapor pressure of solid copper is reported to be 3.33×10-7 atm at 1060 °C. Estimate the number of gaseous copper atoms per cubic centimeter in equilibrium with solid copper at 1060 °C (23 °C below its melting point). atoms/cm3arrow_forwardWhich liquid is expected to have the higher surface tension: octane, C3H18, or 1-octanol, C3H17OH? Explain.arrow_forwardHow much heat energy is required to convert 35.8 g of liquid sulfur dioxide, SO2, at 207.5 K to gaseous SO2 at 263.1K if the molar heat of vaporization of SO2 is 24.9 kJ/mol, and the specific heat capacity (?) of liquid SO2 is 1.36 J/(g·∘C)? Give q in kj.arrow_forward
- Helium condenses to a liquid at 4.224 K under atmospheric pressure and remains a liquid down to the absolute zero of temperature. (It is used as a coolant to reach very low temperatures.) The vapor pressure of liquidhelium at 2.20 K is 0.05256 atm. Calculate the volume occupied by 1.000 mol helium vapor under these conditions and compare it with the volume of the same amount of helium at standard temperature and pressure.arrow_forwardWhich type of intermolecular force accounts for each ofthese differences? (a) CH3OH boils at 65 °C; CH3SH boils at6 °C. (b) Xe is a liquid at atmospheric pressure and 120 K,whereas Ar is a gas under the same conditions. (c) Kr,atomic weight 84 amu, boils at 120.9 K, whereas Cl2, molecularweight about 71 amu, boils at 238 K. (d) Acetone boilsat 56 °C, whereas 2-methylpropane boils at -12 °C.arrow_forwardArgon at a pressure of 1 atm condenses to a liquid at 87.4 K and solidifies at 83.9 K. The vapor pressure of liquid argon is 0.922 atm at 86.6 K Calculate the volume of 1.00 mol of Ar vapor under these conditions and compare it with the volume of 1.00 mol of Ar at STP (0 °C, 1 atm). Volume 1 mol vapor at 86.6 K Volume 1 mol gas at STP. The volume of 1.00 mol gas at STP is larger than the volume of 1.00 mol vapor at 86.6 Karrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning


