
Concept explainers
(a)
Interpretation:
The formal charges of element A if it was in (a) Group 7A, (b) Group 5A, (c) Group 3A should be drawn.
Concept Introduction
- A formal charge (FC) is the charge assigned to an atom in a molecule, irrespective of relative electronegativity by thinking that electrons in all
chemical bonds are shared equally among atoms. - This method is used to identify the most probable Lewis structures if more than one possibility exists for a compound.
- Formal charge of an atom can be determined by the given formula.
To determine: Formal charge of the given species
(a)
Answer to Problem 5PPC
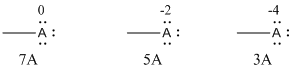
Explanation of Solution
Given Lewis structure
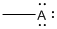
A formal charge (FC) is the charge assigned to an atom in a molecule, irrespective of relative electronegativity.
The formal charge of the given species is calculated,
- If A is group 7A, then number of valence electrons is 7
Substituting these values to the equation,
- If A is group 5A, then number of valence electrons is 5
Substituting these values to the equation,
- If A is group 3A, then number of valence electrons is 3
Substituting these values to the equation,
Therefore,
The formal charges are,
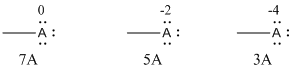
(b)
Interpretation:
The formal charges of element A if it was in (a) Group 7A, (b) Group 5A, (c) Group 3A should be drawn.
Concept Introduction
- A formal charge (FC) is the charge assigned to an atom in a molecule, irrespective of relative electronegativity by thinking that electrons in all chemical bonds are shared equally among atoms.
- This method is used to identify the most probable Lewis structures if more than one possibility exists for a compound.
- Formal charge of an atom can be determined by the given formula.
To determine: Formal charge of the given species
(b)
Answer to Problem 5PPC
Explanation of Solution
Given Lewis structure
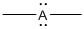
A formal charge (FC) is the charge assigned to an atom in a molecule, irrespective of relative electronegativity.
The formal charge of the given species is calculated,
- If A is group 7A, then number of valence electrons is 7
Substituting these values to the equation,
- If A is group 5A, then number of valence electrons is 5
Substituting these values to the equation,
- If A is group 3A, then number of valence electrons is 3
Substituting these values to the equation,
Therefore,
The formal charges are,

(c)
Interpretation:
The formal charges of element A if it was in (a) Group 7A, (b) Group 5A, (c) Group 3A should be drawn.
Concept Introduction
- A formal charge (FC) is the charge assigned to an atom in a molecule, irrespective of relative electronegativity by thinking that electrons in all chemical bonds are shared equally among atoms.
- This method is used to identify the most probable Lewis structures if more than one possibility exists for a compound.
- Formal charge of an atom can be determined by the given formula.
To determine: Formal charge of the given species
(c)
Answer to Problem 5PPC
Explanation of Solution
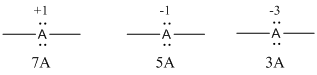
Given Lewis structure
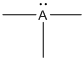
A formal charge (FC) is the charge assigned to an atom in a molecule, irrespective of relative electronegativity.
The formal charge of the given species is calculated,
- If A is group 7A, then number of valence electrons is 7
Substituting these values to the equation,
- If A is group 5A, then number of valence electrons is 5
Substituting these values to the equation,
- If A is group 3A, then number of valence electrons is 3
Substituting these values to the equation,
Therefore,
The formal charges are,

Want to see more full solutions like this?
Chapter 6 Solutions
Chemistry: Atoms First
- Write the Lewis symbol for atoms of each of the following elements:(a) Al, (b) Br, (c) Ar, (d) Sr.arrow_forward8D.4. Benzene, a common solvent, is a covalent molecular compound which contains only carbon and hydrogen. Its simplest (empirical) formula is CH, and its molecular weight is 78 g/mol to 2 significant digits. What is its molecular formula? 8D.5. Draw the Lewis Dot Structure of (a) phosphorus pentafluoride. What is the formal charge on all atoms? (b) the perchlorate ion- in this case expand the octet for the chlorine atom in order to optimize the formal charges.arrow_forwardIn the vapor phase, BeCl2 exists as a discrete molecule. (a) Draw the Lewis structure of this molecule, using only single bonds. Does this Lewis structure satisfy the octet rule? (b) What other resonance structures are possible that satisfy the octet rule? (c) On the basis of the formal charges, which Lewis structure is expected to be dominant for BeCl2?arrow_forward
- (b) Methyl azide, CH-N3 is a molecule that decomposes explosively. Draw a Lewis structure for CH3N3, showing formal charges and also sketch any possible resonance forms.arrow_forwardA stable triatomic molecule can be formed that contains one atom each of nitrogen, sulfur, and fluorine. Three bonding structures are possible, depending on which is the central atom: NSF, SNF, and SFN. (a) Write a Lewis diagram for each of these molecules, indicating the formal charge on each atom. (b) Often, the structure with the least separation of formal charge is the most stable. Is this statement consistent with the observed structure for this molecule— namely, NSF, which has a central sulfur atom? (c) Does consideration of the electronegativities of N, S, and F from Figure 3.18 help rationalize this observed structure? Explain. 100. The gasarrow_forwardWrite Lewis structures for the following: (c) C2F6 (contains a C¬C bond), (d) AsO3 3 -, (e) H2SO3 (H is bonded to O), (f) NH2Cl.. Arrange the bonds in each of the following sets in order of increasing polarity: (a) C¬F, O¬F, Be¬F; (b) O¬Cl, S¬Br, C¬P; (c) C¬S, B¬F, N¬O. What is the Lewis symbol for each of the following atoms or ions? (a) K, (b) As, (c) Sn2 + , (d) N3 Write electron configurations for the following ions and determine which have noble-gas configurations: (a) Cd2+, (b) P3-, (c) Zr4+arrow_forward
- A resident expert on electronegativity comes up to visit with you. He makes two claims (seen below) about electronegativity with relation to covalent bonding. Is the expert correct or can you refute him with your knowledge of electronegativity? (a) If a diatomic molecule is made up of atoms X and Y, which have different electronegativities, the molecule must be polar. (b) The farther two atoms are apart in a bond, the larger the dipole moment will be.arrow_forwardWhich of the following bonds are polar: (a) P—O; (b) S—F; (c) Br—Br; (d) O—Cl? Which is the more electronegative atom in each polar bond?arrow_forwardIncomplete Lewis structures for the nitrous acid molecule,HNO2, and the nitrite ion, NO2-, are shown here. (a) Completeeach Lewis structure by adding electron pairs as needed.(b) Is the formal charge on N the same or different in thesetwo species? (c) Would either HNO2 or NO2- be expected toexhibit resonance? (d) Would you expect the N=O bond inHNO2 to be longer, shorter, or the same length as the N¬Obonds in NO2?arrow_forward
- Keeping in mind that some elements violate the octet rule, draw a Lewis structure for each compound: (a) BeH 2; (b) PCl 5.arrow_forwardIn each case, tell whether the bond is ionic, polar cova- lent, or nonpolar covalent. (a) Br, (e) SiH, (d) SrF, (c) HCl (g) N, (b) BrCl 2 (f) CO (h) CsCl 4.arrow_forwardAlthough I3- is a known ion, F3- is not. (a) Draw the Lewis structure for I3- (it is linear, not a triangle). (b) One of your classmates says that F3 - does not exist because F is too electronegative to make bonds with another atom. Give an example that proves your classmate is wrong. (c) Another classmate says F3- does not exist because it would violate the octet rule.Is this classmate possibly correct? (d) Yet another classmatesays F3- does not exist because F is too small to make bonds tomore than one atom. Is this classmate possibly correct?arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
