
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 10, Problem 10.43P
The tosylate of a primary alcohol normally undergoes an SN2 reaction with hydroxide ion to give a primary alcohol. Reaction of this tosylate, however, gives a compound of molecular formula C7H12O.
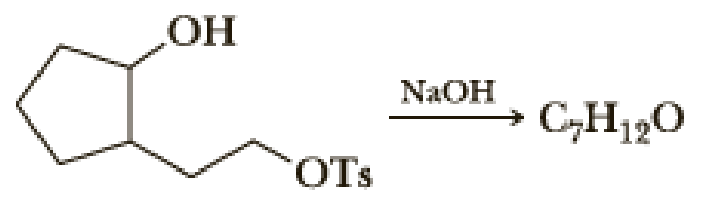
Propose a structural formula for this compound and a mechanism for its formation.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
The tosylate of a primary alcohol normally undergoes an S2 reaction with hydroxide
ion to give a primary alcohol. Reaction of this tosylate, however, gives a compound of
molecular formula C,H,0.
OH
NaOH
C,H190
OTs
Propose a structural formula for this compound and a mechanism for its formation.
Neopentyl alcohol, (CH3)3CCH2OH, reacts with concentrated HBr to give 2-bromo2-methylbutane, a rearranged product. Propose a mechanism for the formation of thisproduct.
When A is reacted with hot aqueous NaOH, a compound B of molecular formula C8H11NO is produced. With this information, write the correct structure of B and propose the reaction mechanism (step by step, with the correct use of arrows) to obtain B.
Chapter 10 Solutions
Organic Chemistry
Ch. 10.1 - Write IUPAC names for these alcohols and include...Ch. 10.1 - Classify each alcohol as primary, secondary, or...Ch. 10.1 - Write IUPAC names for these unsaturated alcohols.Ch. 10.2 - Arrange these compounds in order of increasing...Ch. 10.2 - Prob. 10.5PCh. 10.4 - Predict the position of equilibrium for this...Ch. 10.5 - Show how to convert (R)-2-pentanol to...Ch. 10.6 - Draw structural formulas for the alkenes formed by...Ch. 10.6 - Propose a mechanism to account for this...Ch. 10.7 - Propose a mechanism to account for the following...
Ch. 10.7 - Prob. AQCh. 10.7 - Prob. BQCh. 10.7 - Prob. CQCh. 10.7 - Prob. DQCh. 10.7 - Which step in the reaction would you expect to be...Ch. 10.7 - Prob. FQCh. 10.7 - Prob. GQCh. 10.8 - Prob. 10.11PCh. 10.8 - Prob. AQCh. 10.8 - Prob. BQCh. 10.8 - Prob. CQCh. 10.8 - Why does nature use a reagent as complex as NAD+...Ch. 10.8 - -Hydroxyketones and -hydroxyaldehydes are also...Ch. 10.9 - Write IUPAC names for these thiols.Ch. 10 - Which are secondary alcohols?Ch. 10 - Name each compound.Ch. 10 - Prob. 10.16PCh. 10 - Name and draw structural formulas for the eight...Ch. 10 - Arrange these compounds in order of increasing...Ch. 10 - Arrange these compounds in order of increasing...Ch. 10 - Prob. 10.20PCh. 10 - Prob. 10.21PCh. 10 - Arrange the compounds in each set in order of...Ch. 10 - Prob. 10.23PCh. 10 - The decalinols A and B can be equilibrated using...Ch. 10 - Prob. 10.25PCh. 10 - Select the stronger acid from each pair and...Ch. 10 - Prob. 10.27PCh. 10 - In each equilibrium, label the stronger acid, the...Ch. 10 - Write equations for the reaction of 1-butanol with...Ch. 10 - Write equations for the reaction of 2-butanol with...Ch. 10 - Prob. 10.31PCh. 10 - When (R)-2-butanol is left standing in aqueous...Ch. 10 - Two diastereomeric sets of enantiomers, A/B and...Ch. 10 - Acid-catalyzed dehydration of 3-methyl-2-butanol...Ch. 10 - Show how you might bring about the following...Ch. 10 - Propose a mechanism for the following pinacol...Ch. 10 - Prob. 10.37PCh. 10 - Show how each alcohol or diol can be prepared from...Ch. 10 - Dihydropyran is synthesized by treating...Ch. 10 - Show how to convert propene to each of these...Ch. 10 - Prob. 10.41PCh. 10 - Prob. 10.42PCh. 10 - The tosylate of a primary alcohol normally...Ch. 10 - Prob. 10.44PCh. 10 - Show how to convert cyclohexene to each compound...Ch. 10 - Prob. 10.46PCh. 10 - Ethanol (CH3CH2OH) and dimethyl ether (CH3OCH3)...Ch. 10 - Prob. 10.48PCh. 10 - Prob. 10.49PCh. 10 - Prob. 10.50PCh. 10 - Write the products of the following sequences of...Ch. 10 - Alcohols are important for organic synthesis,...Ch. 10 - Using your reaction roadmap as a guide, show how...Ch. 10 - Using your reaction roadmap as a guide, show how...Ch. 10 - Using your reaction roadmap as a guide, show how...Ch. 10 - Using your reaction roadmap as a guide, show how...Ch. 10 - Prob. 10.57PCh. 10 - Prob. 10.58PCh. 10 - Prob. 10.59P
Additional Science Textbook Solutions
Find more solutions based on key concepts
Consider a sample of ideal gas initially in a volume V at temperature T and pressure P. Does the entropy of thi...
General Chemistry: Principles and Modern Applications (11th Edition)
Practice Problem 1.22 Which of the following alkenes can exist as cis-trans isomers? Write their structures. Bu...
Organic Chemistry
1. What did each of the following scientists contribute to our knowledge of the atom?
a. William Crookes
b. E...
Chemistry For Changing Times (14th Edition)
Practice Exercise 1
Which of the following factors determines the size of an atom? a. the volume of the nucleus...
Chemistry: The Central Science (14th Edition)
Draw a Lewis structure for each of the following species: a. H2CO3 b. CO32 c. CH2O d. CO2
Essential Organic Chemistry (3rd Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Nonconjugated , -unsaturated ketones, such as 3-cyclohexenone, are in an acid-catalyzed equilibrium with their conjugated , -unsaturated isomers. Propose a mechanism for this isomerization.arrow_forwardAldehydes and ketones react with thiols to yield thioacetals just as they react with alcohols to yield acetals. Predict the product of the following reaction, and propose a mechanism:arrow_forwardThe following sequence of steps converts (R)-2-octanol to (S)-2-octanol. Propose structural formulas for intermediates A and B, specify the configuration of each, and account for the inversion of configuration in this sequence.arrow_forward
- When cis-4-chlorocyclohexanol is treated with sodium hydroxide in ethanol, it gives mainly the substitution product trans-1,4-cyclohexanediol (1). Under the same reaction conditions, trans-4-chlorocyclohexanol gives 3-cyclohexenol (2) and the bicyclic ether (3). (a) Propose a mechanism for formation of product (1), and account for its configuration. (b) Propose a mechanism for formation of product (2). (c) Account for the fact that the bicyclic ether (3) is formed from the trans isomer but not from the cis isomer.arrow_forwardBisphenol A is made on a large scale by a condensation of phenol with acetone. Suggest an appropriate catalyst, and propose a mechanism for this reaction.arrow_forwardProvide reaction mechanisms for the following transformationsarrow_forward
- Propose a mechanism for the reaction of(a) 1-methylcyclohexanol with HBr to form 1-bromo-1-methylcyclohexane.(b) 2-cyclohexylethanol with HBr to form 1-bromo-2-cyclohexylethane.arrow_forward2,3-Dimethylbutane reacts with bromine in the presence of light to give a mono brominated product. The further reaction gives a good yield of a dibrominated product. Predict the structures of these products, and propose a mechanism for the formation of the mono brominated product.arrow_forwardWhen diethyl ether (CH3CH2OCH2CH3) is treated with concentrated HBr, the initial products are CH3CH2Br and CH3CH2OH. Propose a mechanism to account for this reaction.arrow_forward
- When the following compound undergoes solvolysis in ethanol, three products are obtained. Propose a mechanism to account for the formation of these products.arrow_forwardIf heated Time4 decomposes giving the elimination products ethane and ethylene (see below) explain how this might be the case. Propose a mechanism for the formation of each of the products. Δ TiMe4 + C2H6arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Coenzymes and cofactors; Author: CH15 SWAYAM Prabha IIT Madras;https://www.youtube.com/watch?v=bubY2Nm7hVM;License: Standard YouTube License, CC-BY
Aromaticity and Huckel's Rule; Author: Professor Dave Explains;https://www.youtube.com/watch?v=7-BguH4_WBQ;License: Standard Youtube License