
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 10.8, Problem 10.12P
α-Hydroxyketones and α-hydroxyaldehydes are also oxidized by treatment with periodic acid.
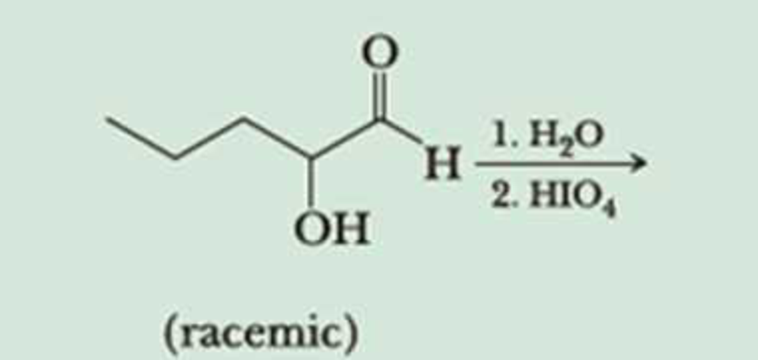
It is not the α-hydroxyketone or
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
The following molecule belongs to a class of compounds called enediols; each carbon of
the double bond carries an-OH group. Draw structural formulas for the a-hydroxyketone
and the a-hydroxyaldehyde with which this enediol is in equilibrium.
CH-OH
a-Hydroxyaldehyde =
C-OH= a -Hydroxyketone
ČH3
An enediol
Prepare the following compounds starting from benzaldehyde and the appropriate ketone. Provide reactions for preparing the ketones starting from aromatic hydrocarbon compounds.
Acetylene reacts with
sodium amide in the
presence of propyl
halide
produces aldehyde
produces ketones
It produces 2-pentane
Chapter 10 Solutions
Organic Chemistry
Ch. 10.1 - Write IUPAC names for these alcohols and include...Ch. 10.1 - Classify each alcohol as primary, secondary, or...Ch. 10.1 - Write IUPAC names for these unsaturated alcohols.Ch. 10.2 - Arrange these compounds in order of increasing...Ch. 10.2 - Prob. 10.5PCh. 10.4 - Predict the position of equilibrium for this...Ch. 10.5 - Show how to convert (R)-2-pentanol to...Ch. 10.6 - Draw structural formulas for the alkenes formed by...Ch. 10.6 - Propose a mechanism to account for this...Ch. 10.7 - Propose a mechanism to account for the following...
Ch. 10.7 - Prob. AQCh. 10.7 - Prob. BQCh. 10.7 - Prob. CQCh. 10.7 - Prob. DQCh. 10.7 - Which step in the reaction would you expect to be...Ch. 10.7 - Prob. FQCh. 10.7 - Prob. GQCh. 10.8 - Prob. 10.11PCh. 10.8 - Prob. AQCh. 10.8 - Prob. BQCh. 10.8 - Prob. CQCh. 10.8 - Why does nature use a reagent as complex as NAD+...Ch. 10.8 - -Hydroxyketones and -hydroxyaldehydes are also...Ch. 10.9 - Write IUPAC names for these thiols.Ch. 10 - Which are secondary alcohols?Ch. 10 - Name each compound.Ch. 10 - Prob. 10.16PCh. 10 - Name and draw structural formulas for the eight...Ch. 10 - Arrange these compounds in order of increasing...Ch. 10 - Arrange these compounds in order of increasing...Ch. 10 - Prob. 10.20PCh. 10 - Prob. 10.21PCh. 10 - Arrange the compounds in each set in order of...Ch. 10 - Prob. 10.23PCh. 10 - The decalinols A and B can be equilibrated using...Ch. 10 - Prob. 10.25PCh. 10 - Select the stronger acid from each pair and...Ch. 10 - Prob. 10.27PCh. 10 - In each equilibrium, label the stronger acid, the...Ch. 10 - Write equations for the reaction of 1-butanol with...Ch. 10 - Write equations for the reaction of 2-butanol with...Ch. 10 - Prob. 10.31PCh. 10 - When (R)-2-butanol is left standing in aqueous...Ch. 10 - Two diastereomeric sets of enantiomers, A/B and...Ch. 10 - Acid-catalyzed dehydration of 3-methyl-2-butanol...Ch. 10 - Show how you might bring about the following...Ch. 10 - Propose a mechanism for the following pinacol...Ch. 10 - Prob. 10.37PCh. 10 - Show how each alcohol or diol can be prepared from...Ch. 10 - Dihydropyran is synthesized by treating...Ch. 10 - Show how to convert propene to each of these...Ch. 10 - Prob. 10.41PCh. 10 - Prob. 10.42PCh. 10 - The tosylate of a primary alcohol normally...Ch. 10 - Prob. 10.44PCh. 10 - Show how to convert cyclohexene to each compound...Ch. 10 - Prob. 10.46PCh. 10 - Ethanol (CH3CH2OH) and dimethyl ether (CH3OCH3)...Ch. 10 - Prob. 10.48PCh. 10 - Prob. 10.49PCh. 10 - Prob. 10.50PCh. 10 - Write the products of the following sequences of...Ch. 10 - Alcohols are important for organic synthesis,...Ch. 10 - Using your reaction roadmap as a guide, show how...Ch. 10 - Using your reaction roadmap as a guide, show how...Ch. 10 - Using your reaction roadmap as a guide, show how...Ch. 10 - Using your reaction roadmap as a guide, show how...Ch. 10 - Prob. 10.57PCh. 10 - Prob. 10.58PCh. 10 - Prob. 10.59P
Additional Science Textbook Solutions
Find more solutions based on key concepts
The active ingredient in Tylenol and a host of other over-the-counter pain relievers is acetaminophen (C8H9NO2)...
Chemistry: Atoms First
The method to determine the volume of a powered solid, liquid and a rock needs to be determined. Concept introd...
Living By Chemistry: First Edition Textbook
Practice Problem ATTEMPT
Write the rate expressions for each of the following reactions:
(a)
(b)
(c)
Chemistry
Write the electron configurations far each of the following elements: (a) Sc. (b) Ti. (c) Cr. (d) Fe. (e) Ru
Chemistry by OpenStax (2015-05-04)
Determine the number of protons, neutrons, and electrons in the following atoms: a. a hydrogen atom that has a ...
General, Organic, and Biological Chemistry (3rd Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 17-60 1-Propanol can be prepared by the reduction of an aldehyde, but it cannot be prepared by the acid catalyzed hydration of an alkene. Explain why it cannot be prepared from an alkene.arrow_forward17-72 The following molecule is an enediol; each carbon of the double bond carries an —OH group. Draw structural formulas for the hydroxyketone and the a-hydroxyaldehyde with which this enediol is in equilibrium.arrow_forward17-35 Suppose that you take a bottle of benzaldehyde (a liquid, bp 179°C) from a shelf and find a white solid in the bottom of the bottle. The solid turns litmus red; that is, it is acidic. Yet aldehydes are neutral compounds. How can you explain these observations?arrow_forward
- Hydration of aldehydes and ketones can be catalyzed by acid or base. Bases catalyze hydration by: protonating the carbonyl oxygen making the carbonyl group more electrophilic employing hydroxide ion, which is a better nucleophile than water making the carbonyl group less electrophilic shifting the equilibrium position of the reaction to favor productsarrow_forwardWhich of the following pairs of reagents are used in the synthesis of the ester below? hexanoic acid + 2-propanol hexanoic acid + 1-propanol propanoic acid + 1-propanol propanoic acid + 2-propanolarrow_forwardAldehydes and ketones react with one molecule of an alcohol to form compounds called hemiacetals, in which there is one hydroxyl group and one ether-like group. Reaction of a hemiacetal with a second molecule of alcohol gives an acetal and a molecule of water. ROH, H+ OH OR Aldehyde/ketone Alcohol ROH, H+ Draw the structural formulas for the hemiacetal and the acetal formed between the following compounds: OH OH OR OR • Use the wedge/hash bond tools to indicate stereochemistry where it exists. + H₂Oarrow_forward
- Give the main organic product for the following reaction: CH2OH OH H3O* CH3CH2CH2OH OH OHarrow_forwardWhen trans-2-chloro-1-cyclohexanol is treated with a base, cyclohexene oxide is the product. However, when cis-2-chloro-1-cyclohexanol is treated with a base, the product is cyclohexanone –arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning
Coenzymes and cofactors; Author: CH15 SWAYAM Prabha IIT Madras;https://www.youtube.com/watch?v=bubY2Nm7hVM;License: Standard YouTube License, CC-BY
Aromaticity and Huckel's Rule; Author: Professor Dave Explains;https://www.youtube.com/watch?v=7-BguH4_WBQ;License: Standard Youtube License