
Concept explainers
(a)
Interpretation:
To predict bond angles about each highlighted carbon.
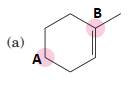
Concept Introduction:
VSEPR predicts the geometry and the bond angles for the molecules according to the theory, the structure is predicted by the lone pairs and bond pairs present in the bond formation.
Electron pair = Lone pair + Bond pair.
| Electron pair | Hybridization | Geometry | Bond angles. |
| 2 | sp | Linear | 180° |
| 3 | sp2 | Trigonal planar | 120° |
| 4 | sp3 | Tetrahedra or square planar | 109.5° or 90° |
| 5 | sp3 d | Trigonal bipyramidal | 120° and 90° |
| 6 | sp3 d2 | Octahedral | 90° |
(b)
Interpretation:
To predict bond angles about each highlighted carbon.
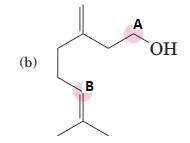
Concept Introduction:
VSEPR predicts the geometry and the bond angles for the molecules according to the theory, the structure is predicted by the lone pairs and bond pairs present in the bond formation.
Electron pair = Lone pair + Bond pair.
| Electron pair | Hybridization | Geometry | Bond angles. |
| 2 | sp | Linear | 180° |
| 3 | sp2 | Trigonal planar | 120° |
| 4 | sp3 | Tetrahedra or square planar | 109.5° or 90° |
| 5 | sp3 d | Trigonal bipyramidal | 120° and 90° |
| 6 | sp3 d2 | Octahedral | 90° |
(c)
Interpretation:
To predict bond angles about each highlighted carbon.
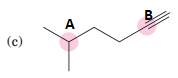
Concept Introduction:
VSEPR predicts the geometry and the bond angles for the molecules according to the theory, the structure is predicted by the lone pairs and bond pairs present in the bond formation.
Electron pair = Lone pair + Bond pair.
| Electron pair | Hybridization | Geometry | Bond angles. |
| 2 | sp | Linear | 180° |
| 3 | sp2 | Trigonal planar | 120° |
| 4 | sp3 | Tetrahedra or square planar | 109.5° or 90° |
| 5 | sp3 d | Trigonal bipyramidal | 120° and 90° |
| 6 | sp3 d2 | Octahedral | 90° |
(d)
Interpretation:
To predict bond angles about each highlighted carbon.
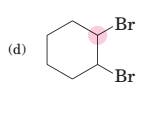
Concept Introduction:
VSEPR predicts the geometry and the bond angles for the molecules according to the theory, the structure is predicted by the lone pairs and bond pairs present in the bond formation.
Electron pair = Lone pair + Bond pair.
| Electron pair | Hybridization | Geometry | Bond angles. |
| 2 | sp | Linear | 180° |
| 3 | sp2 | Trigonal planar | 120° |
| 4 | sp3 | Tetrahedra or square planar | 109.5° or 90° |
| 5 | sp3 d | Trigonal bipyramidal | 120° and 90° |
| 6 | sp3 d2 | Octahedral | 90° |
Want to see the full answer?
Check out a sample textbook solution
Chapter 12 Solutions
Introduction to General, Organic and Biochemistry
- Use the white balls for hydrogen, the black ones with four points of attachment for carbon, and the red ones wit four points of attachment for oxygen, and the blue ones with four points of attachment for nitrogen. Shape H H-C-H H Methane H-N-H H Ammonia Water Harrow_forwardThe carbon skeleton for 2-methylpropane is shown below. How many hydrogens are present? c-d-c 4 9 10 13 14 16arrow_forwardPredict all bond angles about each highlighted carbon atom. b Blue atom bond angles = Red atom bond angles = H3C -CH₂OH H3C Blue atom bond angles ⒸH₂CC=CC=CH₂ Blue atom bond angles = Red atom bond angles = dH3C. CH3 CH3 Blue atom bond angles =arrow_forward
- Predict bond angles about each carbon, oxygen, and nitrogen atom.arrow_forwardProvide the correct IUPAC name for the skeletal (line-bond) structure shown here. * 2,2,3,3-3,3- 2,3- 2,2,2,2- 3,3,3,3- tri di penta tetra eth hex meth 2,2-arrow_forwardC=0 Polar Covalent Choose... None of the above CH3 COOH lonic Non-Polar lonic & Nonpolar Covalen Polar Covalent NH2arrow_forward
- 4a.) Consider the alkane drawn below. Convert this structure into a valid Newman projection, looking down the labeled C₁-C₂ bond. CH3 H3C. C2 C₁ CH3 CH3arrow_forwardSelect the correct value for the indicated bond angle in each of the compounds. 0-0-0 angle of O3 O-S-O angle of SO3 109.5° 120° 120° <109.5° <120° 180° 90° 90° 180° 109.5° <109.5° <120° F-0-F angle of OF2 Cl–Be-Cl angle of BeCl, <120° 180° 120° <109.5° 180° 109.5° <109.5° <120° 109.5° 90° 90° 120° F-P-Fangle of PF3 Cl-Si-Cl angle of SiCl4 <120° 180° 180° 120° <109.5° 109.5° 109.5° <109.5° 90° <120° 120° 90°arrow_forwardEthambutol is a drug used to treat tuberculosis. Determine the shape around the indicated atoms in ethambutol. Don't forget to add lone pairs to heteroatoms before determining the shape around them. CH CH3 CH,CH3 H-O-CH-C-N-CH,CH,-Ņ-C-CH,-0-H H H ethambutol O a. bent, linear, tetrahedral, trigonal pyramid Ob. bent, tetrahedral, tetrahedral. trigonal pyramid O c. linear, linear, linear, trigonal pyramid O d. bent, linear, linear, trigonal pyranid A O E 4) I search hp 近arrow_forward
- To expand on the discussion of carbon valence and hybridization, provide two simple hydrocarbons that have carbon atoms ranging from 3 to 8. Identify the hybridization and bond angles with respect to the carbon atoms. Number the sigma bonds and pi bonds present. Include a drawing of their structure.arrow_forwardDraw the bond-line formula for each of the following condensed structural formulas.These may go beyond the simple explanations above but you can imply the connectivity based on the atoms’ desired valence. a)(CH3)3CCH2CH3 b)(CH3)2CHOH c)(CH3)2CHOHarrow_forward3. Use the VSEPR model to predict the bond angle around the carbon with the arrow. H-C H CIH -CIarrow_forward
 Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning


