
Introduction To General, Organic, And Biochemistry
12th Edition
ISBN: 9781337571357
Author: Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 14, Problem 42P
15-46 Consider Lunesta, a nonbenzodiazepine hypnotic agent (i.e., sleep-inducing drug) that is frequently advertised on TV commercials. Answer the following questions with respect to the given structure:
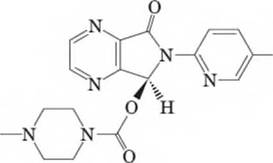
Lunesta
- Determine the molecular formula for Lunesta.
stereocenter(s) and therefore
possible stereoisomer(s). Of the possible
stereocenter(s),
is/are R and
is/are S.
- Does Lunesta have an enantiomer? Does it have a diastereomer?
- Which of the following is true about an enantiomer of Lunesta? Identify all that apply:
Draw an enantiomer of Lunesta.
Examine the derivative of the representation of the six-membered ring found in Lunesta. Draw the alternative chair conformations of this ring and label the more stable chair conformation. (Chapter 11)
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Draw and identity thr hybridized atoms (sp/sp2), draw reaonance structures/ polar bonds, and acidic hydrogens and basic positions for the compound Omeprazole.
Give IUPAC names for the following aromatic compounds:
Benzopyrene, naphthalene and pyrene are members of these group of aromaticcompounds: *Non-benzenoid aromatic compoundsHeterocyclic aromatic compoundsBenzenoid aromatic compoundsHeteronuclear compoundsWhat type of aromatic compound is pyridine? *Benzenoid aromatic compoundNon-benzenoid aromatic compoundHomonuclear cyclic compoundHeterocyclic aromatic compoundWhat property of aromatic rings prevent the involvement of the conjugated structure toaddition reactions? *Radical stabilizationResonance stabilityInductive effectAromatic effect
Chapter 14 Solutions
Introduction To General, Organic, And Biochemistry
Ch. 14.1 - Prob. 14.1QCCh. 14.2 - Problem 15-2 Assign priorities to the groups in...Ch. 14.2 - Problem 15-3 Assign an R or S configuration to the...Ch. 14.3 - Problem 15-4 3-Amino-2-butanol has two...Ch. 14.3 - Prob. 14.5QCCh. 14.3 - Prob. 14.6QCCh. 14 - 15-7 Answer true or false. The cis and trans...Ch. 14 - 15-8 What does the term “chiral” mean? Give an...Ch. 14 - 15-9 What does the term “achiral” mean? Give an...Ch. 14 - 15-10 Define the term “stereoisomer.” Name three...
Ch. 14 - 15-11 In what way are constitutional isomers...Ch. 14 - 15-12 Which of the following objects are chiral...Ch. 14 - Prob. 7PCh. 14 - Prob. 8PCh. 14 - Prob. 9PCh. 14 - Prob. 10PCh. 14 - 15-15 Explain why the carbon of a carbonyl group...Ch. 14 - 15-16 Which of the following compounds contain...Ch. 14 - 15-17 Which of the following compounds contain...Ch. 14 - Prob. 14PCh. 14 - 15-19 Draw the mirror image for each molecule: OH...Ch. 14 - Prob. 16PCh. 14 - 15-21 Answer true or false. For a molecule with...Ch. 14 - Prob. 18PCh. 14 - Prob. 19PCh. 14 - Prob. 20PCh. 14 - Prob. 21PCh. 14 - 15-26 For centuries, Chinese herbal medicine has...Ch. 14 - Prob. 23PCh. 14 - Prob. 24PCh. 14 - Prob. 25PCh. 14 - Prob. 26PCh. 14 - Prob. 27PCh. 14 - Prob. 28PCh. 14 - Prob. 29PCh. 14 - Prob. 30PCh. 14 - 15-35 Following are structural formulas for three...Ch. 14 - Prob. 32PCh. 14 - 15-37 Consider a cyclohexane ring substituted with...Ch. 14 - Prob. 34PCh. 14 - Prob. 35PCh. 14 - Prob. 36PCh. 14 - 15-41 Compound A(C5Hh, is not optically active and...Ch. 14 - Prob. 38PCh. 14 - 15-43 Triamcinolone acetonide, the active...Ch. 14 - 15-44 Consider the structure of the...Ch. 14 - Prob. 41PCh. 14 - 15-46 Consider Lunesta, a nonbenzodiazepine...Ch. 14 - Prob. 43PCh. 14 - Prob. 44P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 16-54 Several poisonous plants, including Atropa belladonna, contain the alkaloid atropine. The name “belladonna” (which means “beautiful lady”) probably comes from the fact that Roman women used extracts from this plant to make themselves more attractive. Atropine is widely used by ophthal mologists and optometrists to dilate the pupils for eye examination. Classify the amino group in atropine as primary, secondary, or tertiary. Locate all stereocenters in atropine. Account for the fact that atropine is almost insoluble in water (1 g in 455 mL of cold water) but atropine hydrogen sulfate is very soluble (1 g in 5 mL of cold water). Account for the fact that a dilute aqueous solution of atropine is basic (pH approximately 10.0).arrow_forward16-28 Following is the structural formula of metformin, the hydrochloride salt of which is marketed as the antidiabetic medication Glucophage. Metformin was introduced into clinical practice in the United States in 1995 for the treatment of type 2 diabetes. More than 25 million prescriptions for this drug were written in 2000, making it the most commonly prescribed brand-name diabetes medication in the nation. NH NH H3(\ 3 N N Nh2ch3 h Metformin Complete the Lewis structure for metformin, showing all valence electrons. Which nitrogen is the most likely site of protonation? Draw the structural formula of Glucophage.arrow_forward16-58 Following is a structural formula of desosamine, a sugar component of several macrolide antibiotics, including the erythromycins. The configuration shown here is that of the natural product. Erythromycin is produced by a strain of Streptomyces erythreus originally found in a soil sample from the Philippine Archipelago. ch3 T Desosamine Name all the functional groups in desosamine. (Chapter 10) How many stereocenters are present in desosamine? How many stereoisomers are possible for it? How many pairs of enantiomers are possible for it? Draw the alternative chair conformations for desosamine and label which groups are equatorial and which are axial. (d > Which of the alternative chair conformations for desosamine is more stable?arrow_forward
- 17-54 Following is the structure of immunosuppressant FK-506, a molecule shown to disrupt calcineurin-mediated signal transduction in T-lymphocytes. (a) There are three carbon—carbon double bonds present in this molecule. Which of the three has the potential for cis/trans isomerism? Assign a cis or trans con?guration to each carbon-carbon double bond that has this possibility. (b) How many stereocenters are present in this molecule? How many stereoisomers are possible for it? (c) Are there any aromatic components in this molecule? (d) Consider the two carbon atoms marked with asterisks. Assign an R or S con?guration of each stereocenter. (e) Because of the presence of a 21-member ring, this molecule is described as a macrocycle. This ring is fashioned by three types of bonds, several carbon-carbon bonds, one ester, one hemiacetal, and one amide. Locate the ester and the hemiacetal. (f) Draw the structural formula of the long chain compound that would result if the hemiacetal were to be cleaved to an alcohol and a carbonyl group.arrow_forward16-17 Propylamine (bp 48°C), ethylmethylamine (bp 37°C), and trimethylamine (bp 3°C) are constitutional isomers with the molecular formula C3HgN. Account for the fact that trimethylamine has the lowest boiling point of the three and propylamine has the highest.arrow_forward13-4 Define aromatic compound.arrow_forward
- Classify the following compounds as aromatic or anti-aromaticarrow_forward18-18 Propanoic acid and methyl acetate are constitutional isomers, and both are liquids at room temperature. One of these compounds has a boiling point of 141°C; the other has a boiling point of 57°C. Which compound has which boiling point? Explain.arrow_forward17-69 Propanal (bp 49°C) and 1-propanol (bp 97°C) have about the same molecular weight, yet their boiling points differ by almost 50°C. Explain this fact.arrow_forward
- 16-19 2-Me thy 1 propane (bp -12°C), 2-propanol (bp 82°C), and 2-propanamine (bp 32°C) all have approximately the same molecular weight, yet their boiling points are quite different. Explain the reason for these differences.arrow_forward17-12 Is it possible for the carbon atom of a carbonyl group to be a stereocenter? Explain.arrow_forward13-7 Can an aromatic compound be a saturated compound?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Acid-Base Titration | Acids, Bases & Alkalis | Chemistry | FuseSchool; Author: FuseSchool - Global Education;https://www.youtube.com/watch?v=yFqx6_Y6c2M;License: Standard YouTube License, CC-BY