
Inorganic Chemistry
5th Edition
ISBN: 9780321811059
Author: Gary L. Miessler, Paul J. Fischer, Donald A. Tarr
Publisher: Prentice Hall
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 4, Problem 4.30P
The structure of 1,1,2,2-tetraiododisilane is shown here. (Reference: T. H. Johansen. K.Hassler. G. Tekautz, K. Hagen, J. Mol. Struct., 2001, 598, 171.)
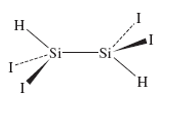
a What is the point group of this molecule?
b. Predict the number of IR-active
c. Predict the number of Raman-active
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Draw a Lewis structure and determine the point group for each of the following molecules. Indicate formal charges for ions. Indicate 3D stereochemistry by drawing solid and hashed bonds. a. XeO3 b. SOF4 c. ClOF2+ d. XeO2F2 e. TeF42-
1. Assign the point group to the following molecules.
* *
co
OH2
2+
H3N,,.
NH3
Mo
H;N co
OH2
H20-Co-CI
H,0
ÓH2
CO
a.
b.
F
H.
d.
f.
Br
CI
OH
CI
Br
OH
g.
h.
Three molecules, BF3, NF3, and CIF3 are all unique in geometry and electronic structure.
a. Using Lewis Structures for the three molecules, determine the point group for all three
molecules.
b. Construct simple MO diagrams for each species
С.
Determine the number of IR and Raman active bands attributed to the M-F bonds for
each molecule
d. Can IR and Raman spectroscopy be used to differentiate between each of the materials?
e. Describe the decoupled F NMR spectra for each of the species
Describe the coupled "F NMR spectra for each of the species (coupling could be to
either B, N, or Cl)
f.
Chapter 4 Solutions
Inorganic Chemistry
Ch. 4.1 - Prob. 4.1ECh. 4.1 - Find all the symmetry elements in the following...Ch. 4.2 - Use the procedure described previously to verify...Ch. 4.3 - Prob. 4.4ECh. 4.3 - Verify the transformation matrices for the E and...Ch. 4.3 - Prepare a representation flowchart according to...Ch. 4.4 - Which point groups are possible for chiral...Ch. 4.4 - Write the corresponding 99 transformation matrices...Ch. 4.4 - Using the x, y, and z coordinates for each atom in...Ch. 4.4 - Reduce the following representations to their...
Ch. 4.4 - Prob. 4.11ECh. 4.4 - Analysis of the x, y, and z coordinates of each...Ch. 4.4 - Determine the number of IR-active CO stretching...Ch. 4.4 - Prob. 4.14ECh. 4 - Determine the point groups for a. Ethane...Ch. 4 - Determine the point groups for a. Ethylene b....Ch. 4 - Determine the point groups for a. Acetylene b....Ch. 4 - Determine the point groups for a. Naphthalene b....Ch. 4 - Determine the point groups for a. 1,1’ ...Ch. 4 - Determine the point groups for a. Cyclohexane...Ch. 4 - Determine the point groups for a. A sheet of...Ch. 4 - Determine the point groups for a. A flat oval...Ch. 4 - Determine the point groups for a. A triangular...Ch. 4 - Determine the point groups for the examples of...Ch. 4 - Determine the point groups of the molecules in the...Ch. 4 - Determine the point groups of the molecules and...Ch. 4 - Determine the point groups of the following atomic...Ch. 4 - a. Show that a cube has the same symmetry elements...Ch. 4 - Suppose an octahedron can have either yellow or...Ch. 4 - What point groups are represented by the symbols...Ch. 4 - Prob. 4.17PCh. 4 - Determine the point groups for the following flags...Ch. 4 - Prepare a representation flowchart according to...Ch. 4 - For trans-1,2-dichloroethylene, which has C2h...Ch. 4 - Ethylene has D2h symmetry. a. List all the...Ch. 4 - Using the D2d character table, a. Determine the...Ch. 4 - Reduce the following representations to...Ch. 4 - For D4h symmetry use sketches to show that dxy...Ch. 4 - Prob. 4.25PCh. 4 - XeOF4 has one of the more interesting structures...Ch. 4 - Repeat the procedure from the previous problem,...Ch. 4 - For the following molecules, determine the number...Ch. 4 - Prob. 4.29PCh. 4 - The structure of 1,1,2,2-tetraiododisilane is...Ch. 4 - Both cis and trans isomers of IO2F4 have been...Ch. 4 - White elemental phosphorus consists of tetrahedral...Ch. 4 - Complexes of the general formula Fe(CO)5x( PR3)x...Ch. 4 - Prob. 4.35PCh. 4 - Prob. 4.36PCh. 4 - Prob. 4.37PCh. 4 - Prob. 4.38PCh. 4 - Determine the point groups of the following...Ch. 4 - Prob. 4.40PCh. 4 - Determine the point groups of the following: a....Ch. 4 - Use the Internet to search for molecules with the...
Additional Science Textbook Solutions
Find more solutions based on key concepts
2. Why shouldn’t you work in a laboratory by yourself?
The Organic Chem Lab Survival Manual: A Student's Guide to Techniques
Practice Problem ATTEMPT
Write the rate expressions for each of the following reactions:
(a)
(b)
(c)
Chemistry
Calculate the lattice energy of CaCl2 using a Born-Haber cycle and data from Appendices F and L and Table 7.5. ...
Chemistry & Chemical Reactivity
During the early part of the 20th century, sulfanilamide (an antibacterial drug) was only administered by injec...
Elementary Principles of Chemical Processes, Binder Ready Version
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Q3: XeF40 belong to the point group C4v, Determine: a. DOF. b. # of vibrations. * c. vibrational modes (vib). [ knowing that FN=4 A₁ + A₂+2B₁ + B₂ + 5E].arrow_forwardTo what point group does the square - pyramidal ion [NI(CN)s] belong? Select one: A. C5v B. D5h С. СAV D. Dad E. D4harrow_forward( A 19. Find the point groups of the following molecules: Bri Br CI G W CI Br Br B Br.... Br CI Br F CI Br C Br... Br Br W CI CI CI D Brassymetric CI Cli Chu..... Br W Br CIarrow_forward
- 升,W4 Symmetry Elements and point group Narme Determine the symmetry elements and assign the point group of: Lewis structures symmetry elements Point group NH:CI Co I Ne rita SiF, Fl.. HCN H-CEN BF. SIFCIBII Cls Br SnF. Fi Sn SeF4 BrFa Fit .. NH #.W4 CI. CI Cllue H:C=CH: H;C-CF: SF. FeBrCh CI etCy Br Cu(NH))4 HyN NH ferrocenearrow_forwardAssign a point group to each of the following compounds and identify how the Pz orbital on the central atom transforms under that respective symmetry. a. NH2Cl b. BF4 - c. HCNarrow_forward4. For each of the following molecules: i. Determine the point group. ii. Determine r (red) as a direct sum of irreducible representations for xyz motion of each atom in the molecule. ii. Determine I (vib) as a direct sum of irreducible representations for molecular vibrations. a. þ,F, b. ХeFsarrow_forward
- 4.30 The structure of 1,1,2,2-tetraiododisilane is shown here. (Reference: T. H. Johansen, K. Hassler, G. Tekautz, K. Hagen, J. Mol. Struct., 2001, 598, 171.) H I- Si -Si I H a. What is the point group of this molecule? b. Predict the number of IR-active Si-I stretching vibrations. c. Predict the number of Raman-active Si-I stretching vibrations.arrow_forward1. Suggest 3 applications of Raman spectroscopy. 2. How polarizability is related to electric dipole moment? 3. How does the induced dipole moment occur? 4. Give the general parts of a Raman spectrophotometer. 5. Raman spectroscopy and infrared spectroscopy measures the vibrational frequencies of a molecule. List the differences between these two spectroscopic techniques.arrow_forwardWrite down all the symmetry elements associated with the following molecules and then use the flow chart to determine the point group of the following molecules. a.) b.) с.) d.) trans-ICrBr,(H,O)l ignore the H's) gauche-CH2CICH2CI В-Н. CH(spiropentane) н. Н Н Н. CI Br o в H.O- OH oosM Н H н Br (triangles Н Н to each other) e.) f.) g.) CHS (thiophene) trans-CFCIBrCFCIBr HCOOH (formic acid) H Вr Cl H-C o-H Br (planar) (planar) k) sulfur hexafluoride ) ethane-Br2Cl2 h) Benzotrifuroxan i dinitrogen tetroxide ) hydrazine P) cyclooctatetraene m) ethylene-BrCl n) 1,I-dichlorethylene o) cyclohexane-Br2Charrow_forward
- 4. For each of the following molecules: i. Determine the point group. ii. Determine (red) as a direct sum of irreducible representations for xyz motion of each atom in the molecule. i. Determine r (vib) as a direct sum of irreducible representations for molecular vibrations. a. B;Hs (diborane)| b. XeF.arrow_forward4. For each of the following molecules: i. þetermine the point group. ii. Determine r (red) as a direct sum of irreducible representations for xyz motion of each atom in the molecule. iii. Determine I (vib) as a direct sum of irreducible representations for molecular vibrations. a. soCI, (thionyl chloride) b. CH,0 (formaldehyde)arrow_forward1. Use the point group assignment flow chart to identify the point group to which each of the following species belongs. (Be sure you can actually identify these, as you'll need to be able to do so on the exam.) a. PH₂ b. PFs c. IFs d. SeF4 e. CF₂Cl₂arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,

Physical Chemistry
Chemistry
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Wadsworth Cengage Learning,
INTRODUCTION TO MOLECULAR QUANTUM MECHANICS -Valence bond theory - 1; Author: AGK Chemistry;https://www.youtube.com/watch?v=U8kPBPqDIwM;License: Standard YouTube License, CC-BY