
Concept explainers
White elemental phosphorus consists of tetrahedral
a. The Raman spectrum of
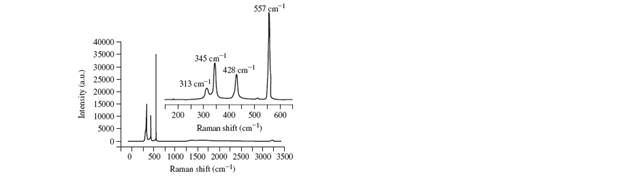
b. If
c. Could a pure sample of
Learn your wayIncludes step-by-step video

Chapter 4 Solutions
Inorganic Chemistry
Additional Science Textbook Solutions
General Chemistry: Principles and Modern Applications (11th Edition)
Chemistry: A Molecular Approach (4th Edition)
Introductory Chemistry (5th Edition) (Standalone Book)
General, Organic, and Biological Chemistry (3rd Edition)
Chemistry: A Molecular Approach
General Chemistry: Atoms First
- (a) What are the C¬C¬C bond angles in diamond?(b) What are they in graphite (in one sheet)? (c) Whatatomic orbitals are involved in the stacking of graphitesheets with each other?arrow_forwardDistinguish between the bond thos exist In CsCl and SiC Crystals.arrow_forwardIdentify the set that contains compounds that form an ionic solid, a molecular solid, and a covalent- network solid. (A) Li2O, B203, BeO; (B) SiO2, P4010, Cl207; (C) Al203, P406, SIO2; (D) CO2, SiO2, GeO2;arrow_forward
- Sodium niobate NaNbO3 is the parent compound of a number of technologically important dielectric materials. For example potassium substitution yields a promising piezoelectric. NaNbO3 is made at high temperatures by reaction of Na₂O and Nb₂O5. The high temperature phase possesses an ideal perovskite structure, while at lower temperatures a number of distorted perovskite polymorphs are observed. (a) With the aid of suitable sketches describe in detail the solid state reaction of Na₂O and Nb₂O5 to form NaNbO3.arrow_forwardCalculate the volume in Å3 of each of the following types ofcubic unit cells if it is composed of atoms with an atomic radiusof 1.82 Å. (a) primitive (b) face-centered cubic.arrow_forwardUse the Born-Mayer equation to estimate the latticeenthalpy of calcium sulfide. CaS. The crystal adopts a rocksalt structure w ith the distance between the centres of nearest neighbours being 284 pm.arrow_forward
- (a) Why does Li Cl acquire pink colour when heated in Li vapours? (b) A solid with cubic crystal is made of two elements ‘P’ and ‘Q’ Atoms of ‘Q’ are at the comers of the cube and ‘P’ at body centre. What is the formula of compound?arrow_forwardWhat is the trend in the size of the bandgap as you move down the column of the group 4A elements?arrow_forwardWhich allotropic form of carbon are conductor and which are non conductor's and why?arrow_forward
- Distinguish between the rock salt structureand the caesium chloride structure for ionicsolidsarrow_forwardGaAs is a compound semiconductor commonly used for LEDs. Talk about the chemical bonds and crystal structures of GaAs crystals and explain why they become semiconductors, not metal alloys (consider the electronic structure of each atom and sp3 hybrid orbit).arrow_forwardWhat is the minimum number of atoms that could be containedin the unit cell of an element with a face-centered cubiclattice? (a) 1, (b) 2, (c) 3, (d) 4, (e) 5.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





