
Concept explainers
What is the major product obtained from the reaction of each of the following compounds with excess HCl?
- a. CH3CH2C≡CH
- b. CH3CH2C≡CCH2CH3
- c. CH3CH2C≡CCH2CH2CH3
(a)
Interpretation:
The major product obtained from the reaction of given compound with excess of
Concept Introduction:
Addition of halides to alkynes: Halogens like chlorine and bromine adds to the alkyne compounds. The general form of the reaction is given below.
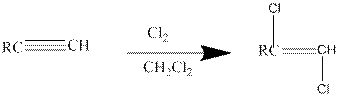
If excess halides are present, a second addition reaction also occurs.
Answer to Problem 29P
The major product obtained from the reaction of given compound with excess of

Explanation of Solution
Hydrogen halides undergo addition reaction with alkynes. Alkyne is a nucleophile and it reacts with the electrophile to give a

In this reaction of butyne with
(b)
Interpretation:
The major product obtained from the reaction of given compound with excess of
Concept Introduction:
Addition of halides to alkynes: Halogens like chlorine and bromine adds to the alkyne compounds. The general form of the reaction is given below.
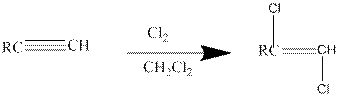
If excess halides are present, a second addition reaction also occurs.
Answer to Problem 29P
The major product obtained from the reaction of given compound with excess of

Explanation of Solution
Hydrogen halides undergo addition reaction with alkynes. Alkyne is a nucleophile and it reacts with the electrophile to give a

In this reaction of hex-3-yne with
(c)
Interpretation:
The major product obtained from the reaction of given compound with excess of
Concept Introduction:
Addition of halides to alkynes: Halogens like chlorine and bromine adds to the alkyne compounds. The general form of the reaction is given below.
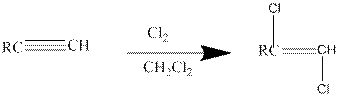
If excess halides are present, a second addition reaction also occurs.
Answer to Problem 29P
The major product obtained from the reaction of given compound with excess of
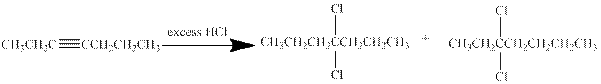
Explanation of Solution
Hydrogen halides undergo addition reaction with alkynes. Alkyne is a nucleophile and it reacts with the electrophile to give a
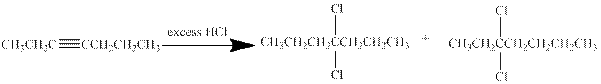
In this reaction of hept-3-yne with
Want to see more full solutions like this?
Chapter 7 Solutions
Organic Chemistry (8th Edition)
- Which solvents favor Sy1 reactions and which favor SN2 reactions? a. CH,CH,OH b. CH,CN c. CH,COOH d. CH,CH2OCH½CH3arrow_forwardThese reagents can produce ketones with alkynes A. BH3, THF, H2O2 B. KMnO4 C. O3 D. H2SO4, H2O, HgSO4arrow_forwardWhat is the major product obtained from the reaction of each of the following compounds with excess HCl? a. CH3CH2C=HC b. CH3CH2C =CCH2CH3 c. CH3CH2C =CCH2CH2CH3arrow_forward
- 2. Provide the major product of each reaction за Li Cl (CH3CH2)2CuLi, -78C 2. H₂O 2 eq.CH3CH2NH2 1. LIALH4 2. HCI, H₂O H nitroethane MeOK, MeOH ii 1.arrow_forwardWhat are the major products of the following reactions? Include stereochemistry. a. 1. Hg(OAc)₂, CH₂OH 2. NaBH4, H₂O, EtOH b. 1. Hg (OAc)₂, (CH3)₂CHOH 2. NaB H₁, H₂O, EtOHarrow_forward6. For the following reactions, predict the major and minor products. Label each. 1. BH₂/THF 2. H₂O₂, OH, H₂O 1. Hg(OAC)₂, H₂O 2. NaBH4arrow_forward
- What is the major organic product obtained from the following sequence of reactions? A. HNO3, H₂SO4 CEN NO₂ B. H₂, Pd/C CN NO₂ Na₂Cr₂O7 H₂SO4 CN D COOH CN 1. NaNO₂, HCI 2. CUCN E CN NO₂arrow_forwardWhich starting material produces the two products shown after ozonolysis and a reductive workup? 1. O3 2. DMS C. А. D. В. A.arrow_forwardWhich compound is the major product of the reaction shown? H9SO, OH C. D. Compound A O Compound B Compound C Compound Darrow_forward
- 2. Draw the major product for each of the following reactions. a. a. Br Br 1.9-BBN 2. H₂O₂, NaOH 1. xs NaNH,/NH3 2. H₂Oarrow_forward7. In eight steps or less, convert the given starting materials into the desired products. eight steps or less a. b. Br CI eight steps or less HO, OHarrow_forward2. Draw the products for the following reaction conditions using the starting material provided. 1. CH3LI; 2. H2O 1. CH3CH2MgBr 2.H20 1.(CH2CH)2CuLi 2. H20arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





