
Concept explainers
(a)
Interpretation:
Synthesis method of the given compound has to be given from the compound which have the same number of carbon atoms.
Concept Introduction:
Addition of water to
Under acidic conditions, alkyne reacts with water to produce an enol which then immediately converts into
The formed enol and ketone are called keto-enol tautomers.
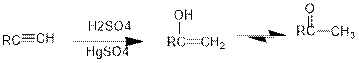
Tautomerization: It is the process of inter conversion of tautomers and therefore the process can also be called as keto-enol tautomerization.
Hydroboration reaction:
It is the reaction in which an internal alkyne is converted into ketone. The reagents used for the hydroboration oxidation reaction is either
(b)
Interpretation:
Synthesis method of the given compound has to be given from the compound which have the same number of carbon atoms.
Concept Introduction:
Addition of water to alkynes in presence of acid:
Under acidic conditions, alkyne reacts with water to produce an enol which then immediately converts into ketone. For terminal alkynes, there is a need of catalyst which is mercury.
The formed enol and ketone are called keto-enol tautomers.
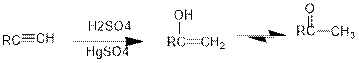
Tautomerization: It is the process of inter conversion of tautomers and therefore the process can also be called as keto-enol tautomerization.
Hydroboration reaction:
It is the reaction in which an internal alkyne is converted into ketone. The reagents used for the hydroboration oxidation reaction is either
(c)
Interpretation:
Synthesis method of the given compound has to be given from the compound which have the same number of carbon atoms.
Concept Introduction:
Addition of water to alkynes in presence of acid:
Under acidic conditions, alkyne reacts with water to produce an enol which then immediately converts into ketone. For terminal alkynes, there is a need of catalyst which is mercury.
The formed enol and ketone are called keto-enol tautomers.
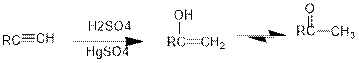
Tautomerization: It is the process of inter conversion of tautomers and therefore the process can also be called as keto-enol tautomerization.
Hydroboration reaction:
It is the reaction in which an internal alkyne is converted into ketone. The reagents used for the hydroboration oxidation reaction is either
Want to see the full answer?
Check out a sample textbook solution
Chapter 7 Solutions
Organic Chemistry (8th Edition)
- What is the name for the following compound? CH3 - CH,CH3 H3C * CH3 a. 4-ethyl-1,3,5-trimethylbenzene O b. 1,3,5-trimethyl-4-ethylbenzene c. 6-ethyl-1,3,5-trimethylbenzene O d. 1-ethyl-2,4,6-trimethylbenzene О е. 2-ethyl-1,3,5-trimethylbenzenearrow_forwardThis type of hydrocarbons contain at least one double or triple bond. Unsaturated hydrocarbons Saturated hydrocarbons Polyunsaturated hydrocarbons O Supersaturated hydrocarbons The most acidic among the hydrocarbons. Alkynes Alkenes Alkanes Arenes The number of secondary carbon(s) in this compound. CH3 CH,-C CHCH, CH3 CIarrow_forward1. Most margarines have been hydrogenated to maintain a soft, homogeneous mixture. While the product is easy to spread, this has made the product an unhealthy choice. A hydrogenation reaction is used to convert an alkene to an alkane. a carboxylic acid to an aldehyde. an alkene to a ketone an aromatic hydrocarbon to a linear hydrocarbonarrow_forward
- Draw the condensed structural formula or skeletal formula, if cyclic, for the alkene that is the major product from each of the following dehydration reactions: H* а. CHз — СН2— СH-— СH2— ОН Heat ОН ОН b. H+ с. H* Heat Нeatarrow_forwardWhich reagent is needed to change an alkyne to an alkane? * Н:0+, Hg (COОH)2 N2, Pt Ог, Pd Н, Pt What reaction takes place when an alcohol is produced during the net addition of water across the double bond of an alkene? * Dehydrogenation Hydrogenation Dehydration Hydrationarrow_forwardDraw the condensed structural formula or skeletal formula, if yclic, for the alkene that is the major product from each of the following dehydration reactions: H+ а. CHз— CH—— СH — СH —ОН - Heat ОН ОН b. H+ с. H+ Heat Heat ОН d. CH3— CH — CH,— СH— СH, Нeat Draw the condensed structural formula for the ether produced y each of the following reactions: H+ а. 2СH3 — ОН Heat H+ b. 2CH3 — СH>— CH-— ОН Heatarrow_forward
- 1. Give the IUPAC names for the following organic compounds. a) CH3CH2CH(CH3)COOH e) CH3CH(CH3)CHBRCOOH ) HOOCCH2CH(CH3)CH2COOH OH h) СООН СООН i) NO2 СООН j) O2N 9 ÇH2CH3 ÇH3 ? HOČCH,CHCH,CH,CHCH,COH k) CH;CH2CHCH,CH;CH3 1) ČO2Harrow_forwardSalim is working on preparation of "Ethers". He wants to prepare Ethoxypropane. He has propanol in the laboratory. What other reagent he needs O Methanol Methane O Ethane O Ethanolarrow_forwardWhich of these compounds is a tertiary alcohol? (Hint: Remember that the classification of alcohols depends on the number of alkyl groups attached to the carbon bonded to the-OH group.) НС- Ң С CH₂ I Н. С OH нес-он CH₂ CH CH3 1 он CH₂arrow_forward
- 4> 39. This aldehyde is useful for its vanilla flavoring hence the trivial name, vanillin. A. B. 40. This important steroid hormone controls the development of male sex characteristics. Cortisone B. Testosterone C Progesterone D. Both B and C 41. The delighted odor of melted butter is attributed to this four-carbon dione. What could be the IUPAC NAME of this ketone? A. Butanone B. Pentanedione C Hexanone D. Butanedione 7arrow_forwardH8. please provide real life applications for following chemical reaction. as well please provide a few example about how to use this reaction in day-to-day life. Reaction: 2-chloro-2-methylpropane + H2O → 2-methylpropan-2-ol + HClarrow_forwardWhich of the following could be the molecular formula for an alkene? A. C7H7 B. C8H16 C. C2H3 D. C2H2 #. C2H6arrow_forward
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning


