
Concept explainers
a)
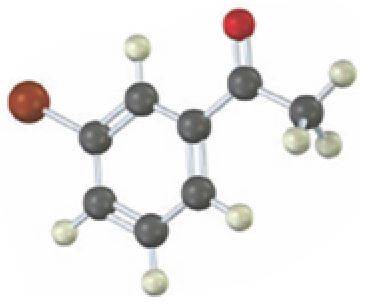
Interpretation:
Starting from which alkyne the compound shown can be prepared is to be stated.
Concept introduction:
Carbonyl compounds can be obtained by the tautomerization of enols produced when
To state:
Starting from which alkyne the compound shown can be prepared.
b)
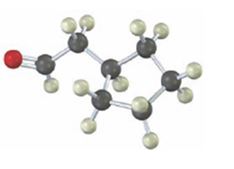
Interpretation:
Starting from which alkyne the compound shown can be prepared is to be stated.
Concept introduction:
Carbonyl compounds can be obtained by the tautomerization of enols produced when alkynes are hydrated. The hydration of terminal alkynes can yield an enol which can tautomerize to an aldehyde or ketone. If oxymercuration- demercuration hydration process is used, the OH adds to the more highly substituted carbon in the triple bond following Markovnokov regiochemistry. If hydroboration –oxidation process is used, the OH adds to the less highly substituted carbon in the triple bond following anti-Markovnokov regiochemistry.
To state:
Starting from which alkyne the compound shown can be prepared.
Want to see the full answer?
Check out a sample textbook solution
Chapter 9 Solutions
Organic Chemistry
- Show how the product formed as a result of the reaction can be synthesized using the starting materials given below. Any reagent needed can be used and you may need more than one step. c) HO, al OH d) b) Онarrow_forwardDraw the structure of the major product for the following two reactions. (PhH = toluene) H H + + X ОН ОН Ph3P=CHCH3 pTsOH (cat) PhHarrow_forward4. Give the starting alkene and any other reagents needed to selectively make the following compounds (with no organic byproducts!): OH L Eto OH all OH Br Br Brarrow_forward
- Show how you are going to synthesize the following products starting with acetylene, CH≡CH, and any other organic or inorganic reagents.arrow_forward2) When conjugated aldehydes or ketones are attacked by a nucleophile, often more than one product forms. Please show resonance structures to explain which positions in the following conjugated ketone can react with NaBH4. Which product(s) can form? + H н-вн Na H CH3OHarrow_forward3) Beginning from acetylene and any alkyl halide needed, how would you prepare the following compound, there may be more than one step. Но HC=CH CHCH3arrow_forward
- What is the major organic product of the following reaction? O HO OH ОН HO O подд олон члон ОН ОН Honlar НО H+ (cat.)arrow_forwardHow can you synthesize the following compound using 2-methylbutane as the starting material?arrow_forwardThe reaction of an alkene with diazomethane forms a cyclopropane ring. Propose a mechanism for the reaction.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





