
Concept explainers
a)
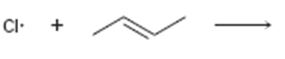
Interpretation:
Curved arrows are to be drawn to show the flow of electrons in the given reaction. The structure of carbon radical that is formed when the halogen radical add to the
Concept introduction:
In reactions involving free radicals homolytic cleavage of covalent bonds takes place. The free radicals produced in the initiation step reacts with the other reactant present in the propagation steps to yield new radicals.
To draw:
Curved arrows to show the flow of electrons in the given reaction and to show the structure of carbon radical that is formed when the halogen radical add to the alkenes.
b)
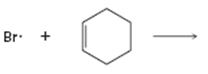
Interpretation:
Curved arrows are to be drawn to show the flow of electrons in the given reaction. The structure of carbon radical that is formed when the halogen radical add to the alkenes is to be drawn.
Concept introduction:
In reactions involving free radicals homolytic cleavage of covalent bonds takes place. The free radicals produced in the initiation step reacts with the other reactant present in the propagation steps to yield new radicals.
To draw:
Curved arrows to show the flow of electrons in the given reaction and to show the structure of carbon radical that is formed when the halogen radical add to the alkenes.
c)
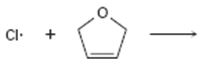
Interpretation:
Curved arrows are to be drawn to show the flow of electrons in the given reaction. The structure of carbon radical that is formed when the halogen radical add to the alkenes is to be drawn.
Concept introduction:
In reactions involving free radicals homolytic cleavage of covalent bonds takes place. The free radicals produced in the initiation step reacts with the other reactant present in the propagation steps to yield new radicals.
To draw:
Curved arrows to show the flow of electrons in the given reaction and to show the structure of carbon radical that is formed when the halogen radical add to the alkenes.
Trending nowThis is a popular solution!

Chapter 6 Solutions
Organic Chemistry
- Zaitsev's rule is useful in selecting which carbon adjacent to a carbocation will form the double bond in the alkene product. True or Falsearrow_forwardA certain hydrocarbon had the molecular formula C18H30 and contained two triple bonds. Ozonolysis gave CH₂(CH₂)CO₂H and HO₂CCH₂CH₂CO₂H as the only products. Draw a reasonable structure for this hydrocarbon. Click and drag to start drawing a structure.arrow_forwardView the first compound name provided in Table 6. Follow the steps below and draw each part of the structure on a piece of paper. Determine the number of carbons present in the compound based on the base name. Draw the carbon chain and include any double or triple bonds if indicated in the suffix of the base name. Number each carbon. The carbons can be numbered from left to right or right to left. Draw any substituents on the corresponding carbon atom for which is indicated in the name. Refer to Figures 3 and 4 in the background for a visual representation of numbered carbons with corresponding substituents. Check that each carbon atom has a total of 4 bonds.arrow_forward
- Draw curved arrows to show the movement of the electrons that result in formation of the given product(s).arrow_forwardDraw the product that would form when 2-methylpropene reacts with chlorine. • You do not have to consider stereochemistry. • You do not have to explicitly draw H atoms. • In cases where there is more than one answer, just draw one. ChemDoodlearrow_forwardA benzene ring alters the reactivity of a neighboring group in the so-called “benzylic” position, similarly to how a double bond alters the reactivity of groups in the “allylic” position. Benzylic cations, anions, and radicals are all more stable than simple alkyl intermediates. a) Use resonance structures to show the delocalization of the positive charge, negative charge, and unpaired electron of the benzyl cation, anion, and radical.arrow_forward
- The compound below is treated with chlorine in the presence of light. CH3 CH3CHCH₂CH3 Draw the structure for the organic radical species produced by reaction of the compound with a chlorine atom. Assume reaction occurs at the weakest C-H bond. • You do not have to consider stereochemistry. • You do not have to explicitly draw H atoms. n [ ]# ?arrow_forwardStart with a cyclohexane. Add double bonds at the 1st, 3rd, and 5th carbons. Add two methyl groups with 1 carbon separating them. Add 1 bromine on the carbon that is in between the two methyls.arrow_forwardDraw the structure(s) of the major organic product(s) of the following reaction. You do not have to consider stereochemistry. • You do not have to explicitly draw H atoms. • Do not include lone pairs in your answer. They will not be considered in the grading. • If no reaction occurs, draw the organic starting material. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. • Separate multiple products using the + sign from the drop-down menu. + Ag₂O/ aqueous THF, 0⁰ TIX. ? ChemDoodleⓇ ▾ < баarrow_forward
- Complete the equation for the reaction between the following Lewis acid-base pair. Use curved arrows to show the flow of electrons in the reaction and draw the product. Assign lone pairs and radical electrons where appropriate. Apply formal charges where appropriate. • Draw the appropriate electron-flow arrows. • Use the "starting points" menu to revert to the original molecule(s) shown. • Omit + signs between structures. ● / CH3 1- H₂C-C CH3 H در St ? ChemDoodleⓇarrow_forwardDraw the structure(s) of the major organic product(s) of the following reaction. H H You do not have to consider stereochemistry. • You do not have to explicitly draw H atoms. • Do not include lone pairs in your answer. They will not be considered in the grading. • If no reaction occurs, draw the organic starting material. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. • Separate multiple products using the + sign from the drop-down menu. 1. LIAIH4 / dry Et₂0 2. aqueous HCI 2 OOD OO. [F ChemDoodleⓇ < 26arrow_forwarda) This alkene can be prepared via Wittig reaction. Draw structural formulas for the aldehyde and the Wittig reagent. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. • Draw the Wittig reagent in the ylide form. • If more than one combination of Wittig reagent and aldehyde is possible, draw only one set. b) How would you convert the alkene to a epoxide? MCPBA c) How many chiral centers are present in the epoxide? V How many stereoisomers are possible in the epoxide that you formed in b)?arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





