
Concept explainers
a)
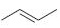
Interpretation:
The reaction of the alkene, 2-butene, with a proton to give a carbocation is to be shown using curved arrows. The structure of the carbocation formed as also to be given.
Concept introduction:
The double bond in
Curved arrows start from a nucleophilic source (neutral or negatively charged) and end in an electrophilic sink (neutral or positively charged). During the flow of the electrons the octet rule must be maintained both in the source and sink.
To show:
Using curved arrows the reaction of the alkene, 2-butene, with a proton to give a carbocation and also to give the structure of the carbocation.
b)
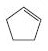
Interpretation:
The reaction of the alkene, cyclopentene, with a proton to give a carbocation is to be shown using curved arrows. The structure of the carbocation formed as also to be given.
Concept introduction:
The double bond in alkenes is nucleophilic. The π electrons can be donated to a proton. By donating the π electrons one of the carbons in the double bond forms a new C-H bond while the other carbon gets a positive charge resulting in a carbocation.
Curved arrows start from a nucleophilic source (neutral or negatively charged) and end in an electrophilic sink (neutral or positively charged). During the flow of the electrons the octet rule must be maintained both in the source and sink.
To show:
Using curved arrows the reaction of the alkene, cyclopentene, with a proton to give a carbocation and also to give the structure of the carbocation.
c)
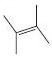
Interpretation:
The reaction of the alkene, 2,3-dimethyl-2-butene, with a proton to give a carbocation is to be shown using curved arrows. The structure of the carbocation formed as also to be given.
Concept introduction:
The double bond in alkenes is nucleophilic. The π electrons can be donated to a proton. By donating the π electrons one of the carbons in the double bond forms a new C-H bond while the other carbon gets a positive charge resulting in a carbocation.
Curved arrows start from a nucleophilic source (neutral or negatively charged) and end in an electrophilic sink (neutral or positively charged). During the flow of the electrons the octet rule must be maintained both in the source and sink.
To show:
Using curved arrows the reaction of the alkene, 2,3-dimethyl-2-butene, with a proton to give a carbocation and also to give the structure of the carbocation.
Trending nowThis is a popular solution!

Chapter 6 Solutions
Organic Chemistry
- Curved arrows are used to illustrate the flow of electrons. Follow the arrows and draw the intermediate and product in this reaction. Include all lone pairs. Ignore stereochemistry. Ignore inorganic byproducts. Draw Intermediate CH₂OH₂* protonation H₂C CH3OH₂* protonation CH3OH deprotonation loss of H₂O elimination Draw Intermediate CH₂OH nucleophilic addition || Draw Intermediate CH3OH deprotonation Draw Productarrow_forward← Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic steps. Be sure to account for all bond-breaking and bond-making steps. Select to Add Arrows Draw Product HBr Select to Add Arrows :Br: Curved arrows are used to illustrate the flow of electrons. Follow the arrows to predict the intermediate and product of this reaction. Include all lone pairs. Use wedges and dashes to indicate stereochemistry. Ignore inorganic byproducts. HCI CH3OH H₂C neutralizing workup -CH₂ Draw Intermediate Problem 27 of 20 Please select a drawing or reagent from the question area Problem 27 of 20 Please select a drawing or reagent from the question areal Problem 4 of 20 Submit Please select a drawing or reagent from the question area Submit Submitarrow_forwardFor the reaction given below, draw a mechanism (curved arrows) and then predict which side of the reaction is favoured under equilibrium conditions.arrow_forward
- In Part 1, draw a mechanism for the reaction of ammonia with butanoic acid. In the box to the left, draw any necessary curved arrows. Show the products of the reaction in the box to the right. Include any nonzero formal charges and all lone pairs of electrons. In Part 2, check the box to indicate which side of the reaction is favored at equilibrium.arrow_forwardDraw the mechaism for the reaction below. Use curved arrows to show electron movement and draw all intermediates.arrow_forwardDraw a curved arrow mechanism for the reaction to form the major product, adding steps as necessary. Be sure to include all nonzero formal charges. U c+ C + C H A H H + Add/Remove step Click and drag to start drawing a structure.arrow_forward
- Compare the following reactions. Which is faster? Using a Free Energy Diagram, justify your choice. Br NaOH Br NaOHarrow_forwardAdd curved arrows to show the forming and breaking of bonds in the reaction below. C с Ċ Add/Remove steparrow_forwardAdd one or more curved arrows to show the movement of electrons in the reaction. To draw the arrows, select More in the drawing menu, then select the appropriate curved arrow. Click on a bond or electron to start a curved arrow. Do not start from an atom. Use the select tool to move the arrow head and tail to the desired placement. When the curved arrow changes from red to black, your arrow has an appropriate starting and ending point. Draw curved arrows. H,C CH, heat H,C-ö. + 0-CHs MacBook Proarrow_forward
- Draw the product(s) of the reaction. Include all lone pairs.arrow_forwardCurved arrows are used to illustrate the flow of electrons. Follow the curved arrows and draw the reactant and product of this hydrohalogenation reaction. Include all lone pairs.arrow_forwardосх (Intermediate) Reactant ķ H H: Tip: Only add curved arrows in this sketcher 1arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





