
Organic Chemistry (9th Edition)
9th Edition
ISBN: 9780321971371
Author: Leroy G. Wade, Jan W. Simek
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 19, Problem 19.50SP
Show how you can synthesize the following compounds starting with benzene, toluene, and alcohols containing no more than four carbon atoms as your organic starting materials. Assume that para is the major product (and separable from ortho) in ortho, para mixtures.
- a. pentan-1-
amine - b. N-methylbutan-1-amine
- c. N-ethyl-N-propylbutan-2-amine
- d. N-benzylpropan-1-amine
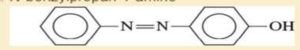
- e. 3-propylaniline
- f. 4-isobutylaniline
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
By means of a series of equations outline a method for carrying out the ff. conversions. You may use
reagents you wish. More than one step maybe required.
a. acetylene
b. cyclohexanone
c. ethyl bromide
d. benzene
athanol
+methylcyclohexanol
acetaldehyde
+phenyl ethanol
B-ethyl-3-pentanol
tyclopentanone
2-суano-2-pentanol
benzyl alcohol
2-butanone-semicarbazone
е. 3-рentanol
f. cyclopentene
g. 2-pentanol
h. benzene
i. 2-butanol
Materials
• 1-butanol
• 2-butanol
• 2-methyl-2-propanol
• 3 mol/L H2SO4 (aq)
• 3 mol/L NaOH
• 0.01 mol/L KMNO4
• concentrated (12 mol/L) HCl (aq) for demonstration use only
• distilled water
• 10 mL graduated cylinders or graduated medicine droppers
• test tubes
• test-tube rack
Procedure
Part 1: The comparison of three isomeric alcohols reactions with potassium permanganate
1. Construct an observation table with the following headings.
Alcohol
Observations at 1 minute
Observations at 5 mins
1-Butanol
2-Butanol
2-Methylpropanol
1. In the reactions involving the three isomeric alcohols with the formula C4H9OH, describewhat each of the following tests showed about reactivity of the -OH group and reactions of 1°,2°, and 3° alcohols.• the test with neutral KMnO4• the test with concentrated HCl2. Predict how the fourth alcohol with the formula C4H10O would react if tested with:• 0.01 M KMnO4• concentrated HCl at room temperatureExtend FurtherUse your observations of the solutions formed in the previous experiments and yourunderstanding of alcohols to complete a table like the one shown below. Research the meltingand boiling points to verify your answers.
Chapter 19 Solutions
Organic Chemistry (9th Edition)
Ch. 19.2A - Prob. 19.1PCh. 19.2B - Prob. 19.2PCh. 19.2B - Give correct names for the following amines:Ch. 19.3 - Prob. 19.4PCh. 19.4 - Prob. 19.5PCh. 19.6 - Rank each set of compounds in order of increasing...Ch. 19.8A - Prob. 19.7PCh. 19.8C - Prob. 19.8PCh. 19.8C - Prob. 19.9PCh. 19.8D - a. Show how fragmentation occurs to give the base...
Ch. 19.10B - Propose a mechanism for nitration of pyridine at...Ch. 19.10B - Prob. 19.12PCh. 19.10C - Prob. 19.13PCh. 19.10C - Prob. 19.14PCh. 19.11 - Propose a mechanism to show the individual...Ch. 19.11 - Prob. 19.16PCh. 19.12 - Give the products expected from the following...Ch. 19.13 - Prob. 19.18PCh. 19.13 - Prob. 19.19PCh. 19.14 - Prob. 19.20PCh. 19.15 - Prob. 19.21PCh. 19.15 - Prob. 19.22PCh. 19.16 - Prob. 19.23PCh. 19.17 - Prob. 19.24PCh. 19.17 - Prob. 19.25PCh. 19.18 - Prob. 19.26PCh. 19.19 - Prob. 19.27PCh. 19.20A - Addition of one equivalent of ammonia to...Ch. 19.20A - Prob. 19.29PCh. 19.20B - Show how you would accomplish the following...Ch. 19.20C - Prob. 19.31PCh. 19 - For each compound, 1. classify the...Ch. 19 - Prob. 19.33SPCh. 19 - Within each structure, rank the indicated...Ch. 19 - In each pair of compounds, select the stronger...Ch. 19 - Which of the following compounds are capable of...Ch. 19 - Complete the following proposed acid-base...Ch. 19 - Predict the products of the following reactions:...Ch. 19 - Prob. 19.39SPCh. 19 - Show how m-toluidine can be converted to the...Ch. 19 - The mass spectrum of tert-butylamine follows shows...Ch. 19 - Prob. 19.42SPCh. 19 - The following drugs are synthesized using the...Ch. 19 - Prob. 19.44SPCh. 19 - Synthesize from benzene. (Hint: All of these...Ch. 19 - Propose mechanisms for the following reactions.Ch. 19 - Prob. 19.47SPCh. 19 - Prob. 19.48SPCh. 19 - Prob. 19.49SPCh. 19 - Show how you can synthesize the following...Ch. 19 - Prob. 19.51SPCh. 19 - The alkaloid coniine has been isolated from...Ch. 19 - A chemist is summoned to an abandoned...Ch. 19 - Pyrrole undergoes electrophilic aromatic...Ch. 19 - Prob. 19.55SPCh. 19 - Prob. 19.56SPCh. 19 - An unknown compound shows a weak molecular ion at...Ch. 19 - A compound of formula C11H16N2 gives the IR,...Ch. 19 - (A true story.) A drug user responded to an ad...Ch. 19 - Prob. 19.60SPCh. 19 - Prob. 19.61SPCh. 19 - Prob. 19.62SPCh. 19 - Prob. 19.63SPCh. 19 - Prob. 19.64SPCh. 19 - Prob. 19.65SP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Suppose you have a mixture of these three compounds. Devise a chemical procedure based on their relative acidity or basicity to separate and isolate each in pure form.arrow_forwardAnswer all three parts plz. You are starting with three aryl bromides. They undergo a typical grignard reaction with magnesium metal and anhydrous ether (diethyl ether). Dry ice is then added to it and it is then cooled. Draw and label the names of the aryl carboxylic acids that will form for each. Part a) bromobenzene Part b) para-bromoanisole Part c) meta-bromoanisolearrow_forward1. Br₂, PBrg 2. H₂O H₂C OH H3C OH Br The a-bromination of carbonyl compounds by Br₂ in acetic acid is limited to aldehydes and ketones because acids, esters, and amides don't enolize to a sufficient extent. Carboxylic acids, however, can be a-brominated by first converting the carboxylic acid to an acid bromide by treatment with PBr3. Following enolization of the acid bromide, Br₂ reacts in an α- substitution reaction. Hydrolysis of the acid bromide completes the reaction. Draw curved arrows to show the movement of electrons in this step of the mechanism. Arrow-pushing Instructions H3C :0: :0::Br: Br Br H3C CO-P H Br Brarrow_forward
- provide the name of the major product(s) of the given reactions. For disubstituted aromatic rings, write the whole word for o-, m-, and p- (ortho-, meta-, and para-). Write your answer in lower caps. 1. phenylacetaldehyde + NaBH4 in the presence of a mild acid 2. p-methylbenzoic acid + Br2 in the presence of FeBr3 3. cyclopentanone + CH3MgBr 4. benzene + 1-chloro-2-methylpentane in the presence of AICI3 5. aniline + Br2 in the presence of FeBr3arrow_forwardWrite the structural formula of the organic product for the given reaction between an alkyne and an alkyl halide. The alkyne group is shown and should be entered as "CC" without the triple bond. Enter C before associated H atoms (e.g., CH3CH2CH2OCHCHCH2). CH3CH2CH2CCH 1. NaNH, 2. CH3CH2Brarrow_forward4. For the following question please consider the four chemicals shown below. Me H2N s, NH2 acetaldehyde Chemical 1 methanamine Chemical 2 S-methyl ethanethioate Chemical 3 ethanamine Chemical 4 Chemical 4 will readily react with two other chemicals above. a. What are those two chemicals? b. Provide a chemical structure of formed products along with a probable mechanism using the arrow formalism. c. At which of the following pH's (out of 1,7,14) do you expect each reaction to occur? Why?arrow_forward
- 4. Which synthesis, starting from benzene, forms aniline (aminobenzene)? a. 1) HNO3/H₂SO4 b. 1) HNO3/H₂SO4 2) H₂, Pd/C c. 1) NH₂/H₂SO4 d. 1) Cl2, FeCl3 2) NaOH, high T,P e. 1) NH₁/H₂SO4 2) H₂, Pd/Carrow_forwardCompound X is insoluble in cold KMnO4, Br2 in CCl4, and conc. H2SO4. Compound X is most likely: a. an alkane b. none of these c. an alkene d. an alcohol e. an alkyl halide Indicate which of the ff. statements regarding nucleophilicity is incorrect. F- is more nucleophilic, hence, more reactive towards methyl iodide than Cl-. Second row elements are more nucleophilic than first row elements of comparable basicity. The rate of SN2 reaction may be markedly affected by the nucleophilicity of the attacking atom. Nucleophilicity is the affinity of a nucleophile to an electrophilic carbon Which of the following alkynes can be deprotonated by NaNH2 in liquid NH3? 3-Methylhex-2-yne Pent-2-yne 3-Methylbutyne none of these Hex-3-ynearrow_forwardShow the structures of the missing substance(s) in each of the following acid-base equilibria, a. CH3-CH2-CH2-NH2 + H2O = 17-42 CH3-CH2-CH2-NH3 + ? b. ?+ H,0 -CH2-NH, + OH" c. CH3-CH-CH2-NH-CH3 + H2O = ? +OH ČH3 d. Trimethylamine + H20 ? +? 17 17-43arrow_forward
- 3. Show how you would synthesises the following compounds starting with benzene, toluene, an alcohols containing no more than four carbon atoms as your starting organic materials. Assume that para is the major product (and separable from ortho) in ortho, para mixture. A. Pentane-1-amine B. N-ethyl-N-propylbutan-2-amine C. N-methylbutan-1-amine D. N-benzylpropan-1-amine -N=N- -OH E.arrow_forwardWhich of the following statements is correct?A. The haloform reaction proceeds under very difficult conditions and the yield is very low.B. The haloform reaction is used only for the identification of compounds containing secondary alcohol groups.C. Methyl ketones or alcohols are oxidized by halogens in acidic solutions to give carboxylic acids and the corresponding haloform product.D. Enolate anions react rapidly with halogens to give alpha-halocarbonyl compounds.arrow_forward5. MULTISTEP SYNTHESIS: Provide a synthesis for the formation of the following compounds using benzene as a starting material and any other reagents you need. Use numbers in your answer for the proper sequence of reagents that you would use. Note: you are only being asked to provide the reagents to prepare each of these compounds from benzene. a. -Brarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Alcohols, Ethers, and Epoxides: Crash Course Organic Chemistry #24; Author: Crash Course;https://www.youtube.com/watch?v=j04zMFwDeDU;License: Standard YouTube License, CC-BY