
Concept explainers
(a)
Interpretation:
The structures of testosterone and estradiol has to be compared.
Concept introduction: Steroids are an example of lipids. They are biologically active organic compounds and are insoluble in water and soluble organic solvents. Example of steroids includes testosterone, estradiol, and cholesterol.
All steroids have common
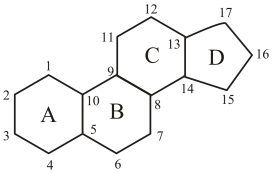
(b)
Interpretation:
The given two structures has to be explained as steroids.
Concept introduction: Steroids are an example of lipids. They are biologically active organic compounds and are insoluble in water and soluble organic solvents. Example of steroids includes testosterone, estradiol, and cholesterol.
All steroids have common

Trending nowThis is a popular solution!

Chapter 24 Solutions
Chemistry & Chemical Reactivity
- (a) Which one of the following is a food preservative?Equanil, Morphine, Sodium benzoate(b) Why is bithional added to soap?(c) Which class of drugs is used in sleeping pills?arrow_forwardAnswer the following :(i) Why is the use of aspartame limited to cold foods and drinks?(ii) How do antiseptics differ from disinfectants?(iii) Why do soaps not work in hard water?arrow_forward(A) Molecule A Molecule B Molecule C Molecule D Molecule E (B) Molecule F (E) Of the molecules depicted above, which two could be the products of an esterification reaction, which is a type of condensation reaction? OH 'OH (C) (D)arrow_forward
- Examine the structural formulas of testosterone (a male sex hormone) and progesterone (a female sex hormone). What are the similarities in structure between the two? What are the differences?arrow_forwardWhich of the following best describes compound 3? It is an… alcohol anhydride thiol amine nitrilearrow_forwardDraw each molecule given its name and the following information. (a) Nitroglycerin, also known as 1,2,3-trinitroxypropane, the active ingredient in dynamite and a medication administered to people having a heart attack, (Hint: The nitroxy group is the conjugate base of nitric acid.)arrow_forward
- ¨ What other types of analgesics are on the market? How does their action compare with aspirin and why are these alternative drugs necessary? Discuss the differences among the following compounds by drawing their structures: Acetylsalicylic acid, Acetaminophen (Paracetamol), Ibuprofen, Paracetamol and Phenacetin.arrow_forwardWhen comparing the structures of aspirin and acetaminophen, why can acetaminophen exist in a liquid state (i.e. childrens drinkable tylenol) and aspirin cannot? Does it have to do with the carboxylic acid? The question says that liquid aspirin cannot be made. Why would that be?arrow_forwardProvide a brief explanation if possible.arrow_forward
- Fats belong to the class of organic compounds represented by the general formula, RCOOR', where R and R' represent hydrocarbon groups; therefore, fats are: a. ethers. b. soaps. c. esters. d. lipases.arrow_forwardFats belong to the class of organic compounds represented by the general formula, RCOOR', where R and R' represent hydrocarbon groups. What is the name of the functional group present in fats? What functional group is common to all saponifiable lipids?arrow_forwardIndicate whether each statement is true or false: (a) Fat molecules contain amide bonds. (b) Phosphoplipids can be zwitterions. (c) Phospholipids form bilayers in water in order to have their long hydrophobic tails interact favorably with each other, leaving their polar heads to the aqueous environment.arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
