
Concept explainers
Interpretation:
The value of
Concept introduction:
For enzymatic reaction, the common equation is given below,
Here,
E is the enzyme.
S is the substrate.
ES is the enzyme substrate complex.
P is the product.
The general equation for Michaelis-Menten is dervied from above equation,
Here,
Take the Reciprocal of both the sides in equation (1),
This equation is called as Lineweaver-Burk equation. By using this equation the value of
Explanation of Solution
The
Given:
The substare concentration and reaction rate are given.
By using the equation,
Calculate the value of
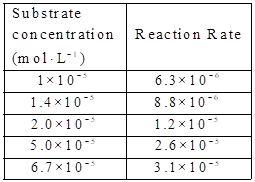
The graph between
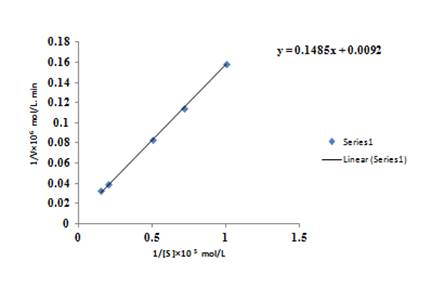
The equation for the straight line obtained from the graph is
Rearrange the equation, in form of
The value of intercept comes from the graph is
Rearrange for
So, the value of
The value of
Want to see more full solutions like this?
Chapter 24 Solutions
Chemistry & Chemical Reactivity
- Fats belong to the class of organic compounds represented by the general formula, RCOOR', where R and R' represent hydrocarbon groups; therefore, fats are: a. ethers. b. soaps. c. esters. d. lipases.arrow_forwardComplete the following statements: a. Oxidation of a secondary alcohol produces ___________. b. Oxidation of a primary alcohol produces an aldehyde that can be further oxidized to a ________. c. Hydrogenation of a ketone produces __________. d. Hydrogenation of an aldehyde produces __________. e. Hydrolysis of an acetal produces _________. f. Hydrolysis of a ketal produces _________.arrow_forwardWhy the nature of oil is not that significant in the process of making of Fatty acid methyl ester in biodiesel synthesis?arrow_forward
- Draw the structural formulas for the first two intermediates formed in the hydrolysis of cis-2,3-epoxybutane with aqueous acid.arrow_forwardEnumerate the uses of esters in pharmacy. What is the purpose of adding concentrated sulfuric acid and heating the mixture? What was the purpose of sodium bicarbonate and sodium chloride solutions in the synthesis of esters?arrow_forwardSalicylic acid is added into a test tube, followed by methanol and sulfuric acid as the catalyst. It is heated for 10-15 minutes and poured into a beaker with crushed ice. This is the esterification of Oil of wintergreen. Write the complete reaction equation and describe the odor of the reactants (salicylic acid and methanol) and the products (oil of wintergreen).arrow_forward
- SYNTHESIS OF ESTERS VIA NUCLEOPHILIC ACYL SUBSTITUTION Write the chemical equation involved in the reaction between the excess acid and NaHCO3. Explain why NaHCO3 is preferred over NaOH for the neutralization of excess acid. How was excess alcohol eliminated from the crude product.arrow_forward7. Write the esterifcation reaction for the synthesis of isopropyl butanoate.arrow_forwardWhat is the role of the acetic acid in the oxidation of Cyclohexanol to Cyclohexanone? Write the balanced chemical reaction between acetic acid and sodium hypochlorite.arrow_forward
- Potassium permanganate and potassium dichromate are very similar in their oxidizing abilities, however there are differences. If I want to convert 4-hexen-1-ol into 4-hexenoic acid, which would be the appropriate oxidizing agent to use? Explain your answer using equations that show the two different products that would form via the two different oxidizing agents.arrow_forwardDraw a structural formula for the intermediate that forms in the acid catalyzed reaction between methanol and 2-ethylbenzoic acid. Give the IUPAC names of the two reagents that would react in a Fischer esterification reaction to produce the intermediate shown. 0-H CH3CH2C- -OCH3 OH reagent 1 = reagent 2 Draw a structural formula for the missing intermediate in the reaction below. H₂SO4 H₂SO4 + CH3OH ? CH3CH2CH2CH2CH2COH + H₂O CH3CH2CH2CH2CH2COCH3arrow_forwardDefine the half-life of synthetic organic compounds?arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning




