
Chemistry & Chemical Reactivity
10th Edition
ISBN: 9781337399074
Author: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Chapter 24.5, Problem 1.3ACP
Interpretation Introduction
Interpretation:
The molecular geometry around the phosphorous atom in a phosphorothioate group has to be identified.
Concept introduction: In phosphorothioate group phosphrous atom bonded with surrounding atoms oxygen and sulphur as shown below.
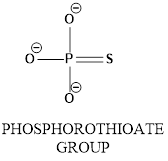
In the diagram shows
Tetrahedral geometry: A tetrahedral has a central atom which is surrounded by four other atoms. In tetrahedral geometry the bond angle is
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
?
Write three difference between ethanol and ethanoic acid on the basis of chemical properties?
This molecule belongs to which class of organic compounds?
CH3⎯CH2⎯O⎯CH3
carboxylic acid
alcohol
aldehyde
ether
ester
Chapter 24 Solutions
Chemistry & Chemical Reactivity
Ch. 24.1 - Draw the Lewis structure for the tripeptide...Ch. 24.3 - What is the sequence of the strand of DNA...Ch. 24.3 - Prob. 24.3CYUCh. 24.5 - Kynamro has the hydrogen bonding sequence:...Ch. 24.5 - The formula of Kynamro is...Ch. 24.5 - Prob. 1.3ACPCh. 24.5 - Prob. 2.1ACPCh. 24.5 - Prob. 2.2ACPCh. 24.5 - Prob. 2.3ACPCh. 24 - (a) Draw the Lewis structure for the amino acid...
Ch. 24 - (a) Draw the Lewis structure for the amino acid...Ch. 24 - Prob. 3PSCh. 24 - Prob. 4PSCh. 24 - Draw Lewis structures for the two dipeptides that...Ch. 24 - Do the amino acid sequences: valine-asparagine and...Ch. 24 - Draw the Lewis structure for the tripeptide...Ch. 24 - Prob. 8PSCh. 24 - Prob. 9PSCh. 24 - Prob. 10PSCh. 24 - Prob. 11PSCh. 24 - Prob. 12PSCh. 24 - (a) Draw the structural formula for the sugar...Ch. 24 - (a) Draw the structural formula for the sugar -D-2...Ch. 24 - Prob. 15PSCh. 24 - Prob. 16PSCh. 24 - Given the following nucleotide sequence in DNA:...Ch. 24 - Given the following nucleotide sequence in DNA: 5'...Ch. 24 - Prob. 19PSCh. 24 - If a drop of oleic acid is added to a dish of...Ch. 24 - What structure do all steroids have in common?Ch. 24 - Prob. 22PSCh. 24 - Prob. 23PSCh. 24 - The chemical equation for the fermentation of...Ch. 24 - Prob. 25PSCh. 24 - Prob. 26PSCh. 24 - Prob. 27GQCh. 24 - Prob. 28GQCh. 24 - Prob. 29GQCh. 24 - Prob. 30GQCh. 24 - Prob. 31GQCh. 24 - There are 41 = 4 mononucleotides of DNA, there are...Ch. 24 - Prob. 33GQCh. 24 - The first step of the metabolic process known as...Ch. 24 - Prob. 35ILCh. 24 - Insulin is a protein important in the metabolism...Ch. 24 - Prob. 37SCQCh. 24 - Prob. 38SCQCh. 24 - Do the DNA sequences ATGC and CGTA represent the...Ch. 24 - Prob. 41SCQCh. 24 - Which of the following statements is/are true? (a)...
Knowledge Booster
Similar questions
- Define the structure of a carboxylic acid ?arrow_forwardExplain the geometry,hybridization and bond angle of carbon atom of a carboxylic acid?arrow_forwardAspirin has the following structure, what are the names of the functional groups present? OH CH3 amide alcohol ether ester ketone aldehyde phenol carboxylic acidarrow_forward
- Draw a structural formula for the following organic compounds: Identify the organic family and the functional group pentanal 3-heptanol propoxybutane 4-nonanone hexanoic acid methyl pentanoatearrow_forwardWrite the chemical equation for a pentanoate ion acting as base when it reacts with hydrochloric acid (HCI). Which is formula of this rule of reaction?arrow_forwardWhich of the following statements correctly describes the typical number of bonds for carbon, nitrogen, and oxygen in most neutral organic molecules?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co