
(a)
Interpretation:
Given molecular compound has to be named.
Concept introduction:
Naming molecular compounds:
To named binary ionic compounds, first predict the positive ion and then negative ion. The positive ion as named as same element while negative ion taking the first part from its original name and ending with –ide.
Numerical prefix for naming compounds are,
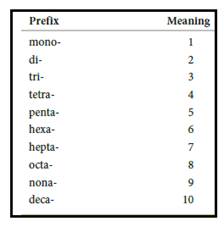
Figure 1
(b)
Interpretation:
The systematic name for given compound has to be given.
Concept introduction:
Naming molecular compounds:
To named binary ionic compounds, first predict the positive ion and then negative ion. The positive ion as named as same element while negative ion taking the first part from its original name and ending with –ide.
Numerical prefix for naming compounds are,

Figure 1
(c)
Interpretation:
The systematic name for given compound has to be given.
Concept introduction:
Naming molecular compounds:
To named binary ionic compounds, first predict the positive ion and then negative ion. The positive ion as named as same element while negative ion taking the first part from its original name and ending with –ide.
Numerical prefix for naming compounds are,
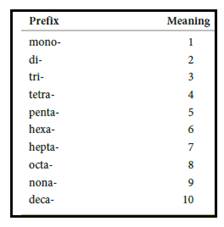
Figure 1
(d)
Interpretation:
The systematic name for given compound has to be given.
Concept introduction:
Naming molecular compounds:
To named binary ionic compounds, first predict the positive ion and then negative ion. The positive ion as named as same element while negative ion taking the first part from its original name and ending with –ide.
Numerical prefix for naming compounds are,
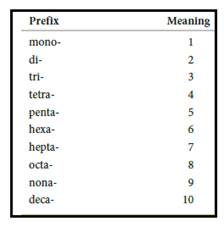
Figure 1
(e)
Interpretation:
The systematic name for given compound has to be given.
Concept introduction:
Naming molecular compounds:
To named binary ionic compounds, first predict the positive ion and then negative ion. The positive ion as named as same element while negative ion taking the first part from its original name and ending with –ide.
Numerical prefix for naming compounds are,

Figure 1
(f)
Interpretation:
Given molecular compound has to be named.
Concept introduction:
Naming molecular compounds:
To named binary ionic compounds, first predict the positive ion and then negative ion. The positive ion as named as same element while negative ion taking the first part from its original name and ending with –ide.
Numerical prefix for naming compounds are,

Figure 1
(g)
Interpretation:
The systematic name for given compound has to be given.
Concept introduction:
Naming molecular compounds:
To named binary ionic compounds, first predict the positive ion and then negative ion. The positive ion as named as same element while negative ion taking the first part from its original name and ending with –ide.
Numerical prefix for naming compounds are,

Figure 1
Want to see the full answer?
Check out a sample textbook solution
Chapter 4 Solutions
General Chemistry: Atoms First
- Name the following covalent compounds:(a) SF6(b) N2O3(c) Cl2O7(d) P4O6arrow_forwardProvide correct names for the following compounds. (a) (b) CI (c) (d) LOCH3 (e) (f) OCH3 Br Br NO2arrow_forwardPredict whether each of the following compounds is molecular or ionic: (a) HClO4(b) CH3OCH3(c) Mg(NO3)2(d) H2Sarrow_forward
- 2. a) b)arrow_forwardGive the name and formula for the acid derived from each of the following anions: (a) perchlorate (b) NO3-(c) bromite (d) H2PO4-arrow_forwardGive the name and formula for the acid derived from each of the following anions: (a) hydrogen carbonate (b) IO4 - (c) cyanide (d) HS-arrow_forward
- A chemist, during the course of an analysis, runs across a chemical composed of carbon, hydrogen, and oxygen in the proportion 1:2:1 and having a six-sided molecular shape. It is probably (a) a pentose, (b) an amino acid, (c) a fatty acid, (d) a monosaccharide, (e) a nucleic acid.arrow_forwardBriefly explain each of the following: (a) Candela (b) Absolute zero (c) P4 (d) Chalcogens (e) An ionic compound (f) A chemical bond (g) An aqueous solution (h) A gas (i) Sublimation (j) Isotopesarrow_forwardWhat is the correct formula for sodium phosphate? (a) Na3PO4(b) Na3P(c) NaPO4(d) Na(PO4)2arrow_forward
- 2N2O5-------> ?(A) 4NO2{g) + O2 (g)(B) 2NO2 + O6(C) N2O6 + O4arrow_forwardName the following compounds. (a) CH,CH3 (b) Et Et Cl CI OH (c) (d) CI Br NO2 Cl 2.arrow_forwardPolyisobutylene is a synthetic elastomer, or rubber. The corresponding monomer is isobutylene, which has the molecular formula C4H8. What is the empirical formula of isobutylene? (A) C4H8 B) C2H4 C2H6 D) CH2arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

