
Organic Chemistry (9th Edition)
9th Edition
ISBN: 9780321971371
Author: Leroy G. Wade, Jan W. Simek
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 10.9D, Problem 10.18P
A formate ester, such as ethyl formate, reacts with an excess of a Grignard reagent to give (after protonation) secondary alcohols with two identical alkyl groups.
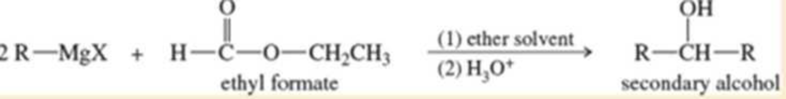
- a. Propose a mechanism to show how the reaction of ethyl formate with an excess of allylmagnesium bromide gives, after protonation, hepta-1,6-dien-4-ol.

- b. Show how you would use reactions of Grignard reagents with ethyl formate to synthesize the following secondary alcohols.
- i. pentan-3-ol
- ii. diphenylmethanol
- iii. trans,trans-nona-2,7-dien-5-ol
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Which of the provided schemes can be used to
synthesize p-chlorophenol
p-chlorophenol from benzen
e?
A. None of the below schemes are
correct
B.
O C.
D.
SO3
H₂SO4
Cl₂
FeCl
903
H₂SO4
Product
Product
Product
1. NaOH
2. H₂O*
SO 3
H₂SO4
Cl₂
FeCl
Product
Product
Product
Cl₂
FeCl
H₂/Pd
1. NaOH
2. H₂O*
p-Chlorophenol
p-Chlorophenol
p-Chlorophenol
What products are obtained from the following reactions?
a. ethyl benzoate + excess isopropanol + HCl b. phenyl acetate + excess ethanol + HCl
Choose the reagent(s) that would be most likely to
complete this reaction.
HILL
Y....
A
B
C
D
E
BH3-THF
H2O2, NaOH
tBuOK
CHCI 3
CH2I2
Zn/Cu
mCPBA
1. Hg(OAc)2, H₂O
2. NaBH4, NaOH
Chapter 10 Solutions
Organic Chemistry (9th Edition)
Ch. 10.3A - Prob. 10.1PCh. 10.3B - Give both the IUPAC name and the common name for...Ch. 10.3B - Prob. 10.3PCh. 10.3C - Give a systematic (IUPAC) name for each diol. a....Ch. 10.4B - Predict which member of each pair will be more...Ch. 10.4B - Dimethylamine (CH3)2NH, has a molecular weight of...Ch. 10.6A - Prob. 10.7PCh. 10.6A - Prob. 10.8PCh. 10.6C - A nitro group (NO2) effectively stabilizes a...Ch. 10.6C - Prob. 10.10P
Ch. 10.8B - Prob. 10.11PCh. 10.8B - Prob. 10.12PCh. 10.9A - Prob. 10.13PCh. 10.9B - Prob. 10.14PCh. 10.9C - Show how you would synthesize each tertiary...Ch. 10.9D - Prob. 10.16PCh. 10.9D - Show how you would add Grignard reagents to acid...Ch. 10.9D - A formate ester, such as ethyl formate, reacts...Ch. 10.9E - Prob. 10.19PCh. 10.9E - In Section9-7B, we saw how acetylide ions add to...Ch. 10.9F - Prob. 10.21PCh. 10.10A - Prob. 10.22PCh. 10.10B - Prob. 10.23PCh. 10.11B - Predict the products you would expect from the...Ch. 10.11B - Prob. 10.25PCh. 10.11B - Prob. 10.26PCh. 10.12 - Prob. 10.27PCh. 10.12 - Prob. 10.28PCh. 10.12 - Authentic skunk spray has become valuable for use...Ch. 10 - Give a systematic (IUPAC) name for each alcohol....Ch. 10 - Give systematic (IUPAC) names for the following...Ch. 10 - Draw the structures of the following compounds...Ch. 10 - Predict which member of each pair has the higher...Ch. 10 - Predict which member of each pair is more acidic,...Ch. 10 - Predict which member of each group is most soluble...Ch. 10 - Draw the organic products you would expect to...Ch. 10 - Prob. 10.37SPCh. 10 - Show how you would synthesize the following...Ch. 10 - Show how you would use Grignard syntheses to...Ch. 10 - Show how you would accomplish the following...Ch. 10 - Show how you would synthesize the following: a....Ch. 10 - Complete the following acid-base reactions. In...Ch. 10 - Prob. 10.43SPCh. 10 - Prob. 10.44SPCh. 10 - Geminal diols, or 1,1-diols, are usually unstable,...Ch. 10 - Vinyl alcohols are generally unstable, quickly...Ch. 10 - Compound A (C7H11Br) is treated with magnesium in...Ch. 10 - Prob. 10.48SPCh. 10 - Prob. 10.49SPCh. 10 - Prob. 10.50SPCh. 10 - Prob. 10.51SPCh. 10 - Prob. 10.52SPCh. 10 - Prob. 10.53SPCh. 10 - Prob. 10.54SPCh. 10 - Prob. 10.55SPCh. 10 - Prob. 10.56SPCh. 10 - Show how this 1 alcohol can be made from the...Ch. 10 - Prob. 10.58SPCh. 10 - Prob. 10.59SPCh. 10 - Prob. 10.60SP
Additional Science Textbook Solutions
Find more solutions based on key concepts
Practice Problem 1.22 Which of the following alkenes can exist as cis-trans isomers? Write their structures. Bu...
Organic Chemistry
The method to determine the volume of a powered solid, liquid and a rock needs to be determined. Concept introd...
Living by Chemistry
141. Design a device that uses as electrochemical cell to determine amount of
in a sample water Describe, in...
Chemistry: Structure and Properties (2nd Edition)
Practice Exercise 1
Which of the following factors determines the size of an atom? a. the volume of the nucleus...
Chemistry: The Central Science (13th Edition)
Describe the orbitals used in bonding and the bond angles in the following compounds: a. CH3O b. CO2 c. H2CO d....
Organic Chemistry (8th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Provide the product most likely to form from reaction of each reagent with 1-methylcyclohexene. You may ingore stereochemistry for this question. Br2 H-Br H-Br, PhC(O)OOC(O)Ph N-bromosuccinimide, PhC(O)OOC(O)Ph A. B. C. D.arrow_forwardWhat is the major product of the reaction of 1 mol of propyne with each of the following reagents? a. HBr (1 mol) e. aqueous H2SO4, HgSO4 h. H2/Lindlar catalyst b. HBr (2 mol) f. R2BH in THF followed by i. sodium amide c. Br2 (1 mol)/CH2Cl2 H2O2/HO-/H2O j. the product of part i followed by d. Br2 (2 mol)/CH2Cl2 g. excess H2, Pd/C 1-chloropropanearrow_forward3. Predict the major product and provide a mechanism for the following: 1-(cyclopentyl-1-ol)-cyclopentanol and sulfuric acid a. b. 2-methyl-1,2-butanediol and sulfuric acid c. 3-cyclopentyl-1,2-propanediol and sulfuric acidarrow_forward
- Grignard reagents react with oxirane (ethylene oxide) to form 1° alcohols but can be prepared in tetrahydrofuran solvent. Why is this difference in behavior observed? A. There is a better leaving group in the oxirane molecule. B. Steric hindrance in the case of tetrahydrofuran precludes reaction with the Grignard. C. It is easier to obtain tetrahydrofuran in anhydrous condition. D. Oxirane is a cyclic ether, while tetrahydrofuran is a hydrocarbon. E. The oxirane ring is the more highly strained.arrow_forwardWhat is the best set of reagents to achieve deoxygenation of 2-pentanone to pentane? A. NaClO2/NaH2PO4 B. LiAlH4, Et2O C. DIBAL-H, THF D. NH2NH2/t-BuOK, DMSOarrow_forward17. Provide the reagents necessary to carry out the 18. Predict the product(s) for the following conversion. following reaction. A. B. C. D. E. NaBH4/CH3OH Na/NH3 1. 2. LIAIH4 H₂O H3O+/heat A and C OH C(CH₂) 141 CH₂OC(CH₂) 14CH3 HOOL CHOC(CH2) 16CH3 CH₂OC(CH2) 14CH3 (CH₂)14C NaOH excess Aarrow_forward
- 2. Provide either the reagents or the major organic product of each reaction, using reactions and reagents we have studied in this course One step A. B. G D. Br =H 1.03 2. Zn, acetic acid reagents?? Cl₂ (=arrow_forward4. i. Define Hemiacetal, Acetal and Ketal. ii. Give the structure of the product from the reaction of propanal with 1M ethanol in dry acid. iii. What happens when Further 1M of ethanol is added to above?arrow_forwardWhich is the best method for the following transformation? OH A. 1. BH3, THF 2. H₂O₂, OH- B. H¹, H₂O C. concentrated H₂SO4 D. 1. Hg(OAc)2, H₂O 2. NaBH4arrow_forward
- Synthesize the following compound from cyclohexanol using any other organic or inorganic compounds. HO. Part 1 out of 2 Preparation of the Grignard reagent: Book rint rences OH draw structure ... draw structure... Draw the intermediate product formed above and select the correct reagent A. O MgBr, O CH;Br O Brz PB13 Draw the intermediate product formed above and select the correct reagent B. O MgBr O Mgo O O Mgl, O Mg Hint Prev 3 of .8 Next > re to searcharrow_forwardWhich alkyl halide would you expect to react most slowly when heated in aqueous solution? å. tert-butyl iodide b. tert-butyl fluoride C. tert-butyl bromide d. tert-butyl chloride Identify the sequence of curved arrows (electron movement) in the steps of the following reaction. :Br: loss of leaving group, rearrangement b. proton transfer, nucleophilic attack loss of leaving group, proton transfer d. loss of leaving group, nucleophilic attackarrow_forwardThis synthesis scheme was designed using the reaction roadmaps. (Reaction roadmaps allow you to "navigate" between the different functional groups and their behavior in chemical reactions.) compound c compound b ● W compound a compound f MAVI compound e m compound d Reagents HBr HBr, H₂O₂, hv a. b. c. H₂O, H₂SO4 d. Br₂ e. Cl₂ f. H₂, Pd Br₂, H₂O Cl₂, H₂O OsO4 then NaHSO3 #[ ] در g. h. i. j. • Use the wedge/hash bond tools to indicate stereochemistry where it exists. • If there are alternative structures, draw the most stable one. • If the reaction produces a racemic mixture, draw both stereoisomers. Separate structures with + signs from the drop-down menu. k. I. m. n. O. p. Hg(OAc)2, H₂O then NaBH4 BH3 then H₂O₂, NaOH O3 then (CH3)2S In this synthesis, reagents from the table are used to carry out the indicated steps (shown in blue). In the box below, draw the structure of compound d. q. r. 2 equivalents of NaNH₂ H2, Lindlar's catalyst Na/NH3 H₂SO4, HgSO4 (sia)2BH then H₂O₂, NaOH 1…arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Alcohols, Ethers, and Epoxides: Crash Course Organic Chemistry #24; Author: Crash Course;https://www.youtube.com/watch?v=j04zMFwDeDU;License: Standard YouTube License, CC-BY