
Organic Chemistry (9th Edition)
9th Edition
ISBN: 9780321971371
Author: Leroy G. Wade, Jan W. Simek
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 8.13, Problem 8.31P
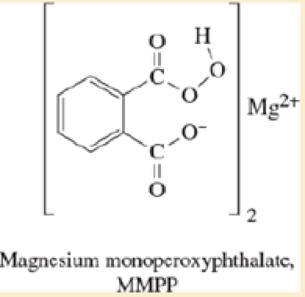
Magnesium monoperoxyphthalate (MMPP)
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Alkylation of benzene with 1-chlorobutane in the presence of AlCl3 gave not only the expected butylbenzene product but also, as a major product, (1-methylpropyl)benzene.
Write an equation for the reaction
Propose a mechanism to account for the formation of butylbenzene
Propose a mechanism to account for the formation of (1-methylpropyl)benzene
In an attempt to prepare propylbenzene, a chemist alkylated benzene with 1-chloropropane and aluminum chloride. However, two isomeric hydrocarbons were obtained in the proportion of 2: 1. What is the main product? How did this come about? Explain showing the mechanism and nomenclature of the reaction compounds
Carototoxin is a natural pesticide produced by carrots, with formula C₁H2O. It undergoes hydrogenation with Pd to:
give product A, C,H,O, and with Lindlar's catalyst to give product B, C,H2O. Ozonolysis followed by zinc leads to a
mixture of methanal, octanal, 1,2-ethanedioic acid, 3-oxopropanoic acid, and 2-hydroxy-3-oxopropanoic acid. Draw
possible structures for carototoxin, A, and B.
Product B structure
H2, Lindlar's
Structure of
carototoxin
H2, Pd/C
Product A structure
Chapter 8 Solutions
Organic Chemistry (9th Edition)
Ch. 8.3A - Predict the major products of the following...Ch. 8.3A - a. When 1 mole of buta-1,3-diene reacts with 1...Ch. 8.3B - Predict the major products of the following...Ch. 8.3B - Show how you would accomplish the following...Ch. 8.4B - Propose a mechanism to show how...Ch. 8.4B - Predict the products of the following hydration...Ch. 8.6 - a. Propose a mochansm fortho following reaction....Ch. 8.6 - Prob. 8.8PCh. 8.6 - Prob. 8.9PCh. 8.7A - Prob. 8.10P
Ch. 8.7A - Prob. 8.11PCh. 8.7C - Prob. 8.12PCh. 8.7C - Prob. 8.13PCh. 8.7C - a. When (Z)-3-methylhex-3-ene undergoes...Ch. 8.7C - Prob. 8.15PCh. 8.7C - Prob. 8.16PCh. 8.8B - Prob. 8.17PCh. 8.8B - Prob. 8.18PCh. 8.9 - Prob. 8.19PCh. 8.9 - Prob. 8.20PCh. 8.9 - Prob. 8.21PCh. 8.9 - Prob. 8.22PCh. 8.10 - Prob. 8.23PCh. 8.10 - Prob. 8.24PCh. 8.10 - Prob. 8.25PCh. 8.11A - Prob. 8.26PCh. 8.11B - Prob. 8.27PCh. 8.11B - Prob. 8.28PCh. 8.12 - Prob. 8.29PCh. 8.13 - a. Propose a mechanism for the conversion of...Ch. 8.13 - Magnesium monoperoxyphthalate (MMPP) epoxidizes...Ch. 8.13 - Predict the major products of the following...Ch. 8.13 - When 1,2-epoxycyclohexane (cyclohexene oxide) is...Ch. 8.14C - Predict the major products of the following...Ch. 8.14C - Prob. 8.35PCh. 8.15B - Prob. 8.36PCh. 8.15C - Predict the major products of the following...Ch. 8.16A - Prob. 8.38PCh. 8.16A - Prob. 8.39PCh. 8.16B - Prob. 8.40PCh. 8.16B - Prob. 8.41PCh. 8.16C - Prob. 8.42PCh. 8.17B - Prob. 8.43PCh. 8.17B - Prob. 8.44PCh. 8.17B - Show how you would synthesize each compound,...Ch. 8 - Prob. 8.46SPCh. 8 - Prob. 8.47SPCh. 8 - Give the products expected when the following...Ch. 8 - Show how you would make the following compounds...Ch. 8 - Using 1,2-dimethylcyclohexene as your starting...Ch. 8 - Show how you would synthesize each compound using...Ch. 8 - Prob. 8.52SPCh. 8 - Show how you might use olefin metathesis to...Ch. 8 - Prob. 8.54SPCh. 8 - Prob. 8.55SPCh. 8 - Propose mechanisms consistent with the following...Ch. 8 - Prob. 8.57SPCh. 8 - Prob. 8.58SPCh. 8 - Draw a reaction-energy diagram for the propagation...Ch. 8 - Prob. 8.60SPCh. 8 - Prob. 8.61SPCh. 8 - Prob. 8.62SPCh. 8 - Prob. 8.63SPCh. 8 - Prob. 8.64SPCh. 8 - Prob. 8.65SPCh. 8 - Prob. 8.66SPCh. 8 - Prob. 8.67SPCh. 8 - Prob. 8.68SPCh. 8 - Prob. 8.69SPCh. 8 - Prob. 8.70SPCh. 8 - Prob. 8.71SPCh. 8 - Prob. 8.72SPCh. 8 - Prob. 8.73SPCh. 8 - Prob. 8.74SPCh. 8 - Prob. 8.75SPCh. 8 - Prob. 8.76SPCh. 8 - Prob. 8.77SPCh. 8 - Prob. 8.78SPCh. 8 - Prob. 8.79SP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Nonconjugated , -unsaturated ketones, such as 3-cyclohexenone, are in an acid-catalyzed equilibrium with their conjugated , -unsaturated isomers. Propose a mechanism for this isomerization.arrow_forwardEnamines normally react with methyl iodide to give two products: one arising from alkylation at nitrogen and the second arising from alkylation at carbon. For example, Heating the mixture of C-alkylation and N-alkylation products gives only the product from C-alkylation. Propose a mechanism for this isomerization.arrow_forwardAmines are converted into alkenes by a two-step process called Hofmann elimination. SN2 reaction of the amine with an excess of CH3I in the first step yields an intermediate that undergoes E2 reaction when treated with silver oxide as base. Pentylamine, for example, yields 1-pentene. Propose a structure for the intermediate, and explain why it readily undergoes elimination.arrow_forward
- Terpin, prepared commercially by the acid-catalyzed hydration of limonene, is used medicinally as an expectorant for coughs. (a) Propose a structural formula for terpin and a mechanism for its formation. (b) How many cis, trans isomers are possible for the structural formula you propose?arrow_forwardAldehydes and ketones react with thiols to yield thioacetals just as they react with alcohols to yield acetals. Predict the product of the following reaction, and propose a mechanism:arrow_forwardThe following sequence of steps converts (R)-2-octanol to (S)-2-octanol. Propose structural formulas for intermediates A and B, specify the configuration of each, and account for the inversion of configuration in this sequence.arrow_forward
- The optically active (2R)-2-phenyl-2-butanol reacts in hydrochloric acid to form haloalkanes and alkenes. The substitution reaction is reported to occur with 100% racemization. Give the structures of the enantiomers that form during the substitution and indicate how much of each is formed. Propose a mechanism for the substitution reaction to yield the R product. What reagents would you use to achieve 100% retention of configuration. Two geometric isomers are obtained during the elimination reaction. Explain mechanistically which alkene will be the main product.arrow_forwardCH3 Ph3P-CHCH3 H3C H3C Aldehydes and ketones are converted into alkenes by means of a direct nucleophilic addition called the Wittig reaction. In the reaction, a triphenylphosphorine ylide, also called a phosphorane, adds to an aldehyde/ketone to give a four-membered cyclic intermediate called an oxaphosphetane. The oxaphosphetane is not isolated but instead spontaneously decomposes to release triphenylphosphine oxide and an alkene. Ph3P-CHCH3 H3C The ylide is formed by reaction of triphenylphosphine, a good nucleophile, with a primary alkyl halide in an S 2 reaction, followed by deprotonation of the carbon with a strong base, such as butyllithium. The carbonyl carbon and the carbon originally bonded to the halogen become the two carbons with the double bond in the product alkene :0: CH3 Com The real value of the Wittig reaction lies in its ability to yield an alkene of predictable structure, as the C-C bond is precisely where the C=O bond was in the reactant and no isomers (other than…arrow_forwardCompound A (C7H11Br) is treated with magnesium in ether to give B (C7H11MgBr) which reacts violently with D2O to give 1-methylcyclohexene with a deuterium atom on the methyl group (C). Reaction of B with acetone (Ch3COCH3) followed by hydrolysis gives D (C10H18O). Heating D with concentrated H2SO4 gives E (C10H16), which reacts with 2 equivalents of Br2 to give F (C10H16Br4). E undergoes hydrogenation with excess H2 and Pt catalyst to give 2-methylpropylcyclohexane. Determine the structures of compounds A through F, and show your reasoning throughout.arrow_forward
- Treatment of 2-bromo-2-methylbutane with KOH in ethanol yields a mixture of two Alkenes products. What are their likely structures? Show its mechanism.arrow_forwardAn optically active monoterpene (compound A) with molecular formula C10H18O undergoes catalytic hydrogenation to form an optically inactive compound with molecular formula C10H20O (compound B). When compound B is heated with acid, followed by reaction with O3 and then with dimethyl sulfide, one of the products obtained is 4-methylcyclohexanone. Give possible structures for compounds A and B.arrow_forwardPlease give the main substitution product for each of the following reactions, and indicate the dominant mechanism: (a) 1-bromopropane + NaOCH3 → (b) 3-bromo-3-methylpentane + NaOC2H5 →arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Coenzymes and cofactors; Author: CH15 SWAYAM Prabha IIT Madras;https://www.youtube.com/watch?v=bubY2Nm7hVM;License: Standard YouTube License, CC-BY
Aromaticity and Huckel's Rule; Author: Professor Dave Explains;https://www.youtube.com/watch?v=7-BguH4_WBQ;License: Standard Youtube License