
Concept explainers
(a)
Interpretation:
The structure for the compound isobutryaldehyde is to be drawn.
Concept introduction:
IUPAC stands for International Union of Pure and Applied Chemistry. The IUPAC gave certain rules which are used worldwide to name the organic compounds. Due to IUPAC naming, each compound has a unique name which is different from their common name.
Answer to Problem 19.1P
The structure of isobutrylaldehyde is shown below.
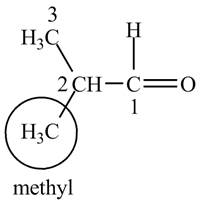
Explanation of Solution
Isobutrylaldehyde is a compound containing 4 carbon atoms and
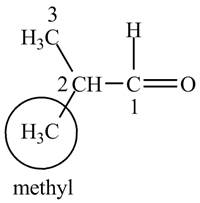
Figure 1
The IUPAC name of the compound is
The structure for the compound isobutrylaldehyde is shown in Figure 1.
(b)
Interpretation:
The structure for the compound valerophenone is to be drawn.
Concept introduction:
IUPAC stands for International Union of Pure and Applied Chemistry. The IUPAC gave certain rules which are used worldwide to name the organic compounds. Due to IUPAC naming, each compound has a unique name which is different from their common name.
Answer to Problem 19.1P
The structure of valerophenone is given below.
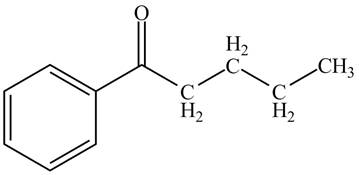
Explanation of Solution
Valerophenone is the name of the compound having

Figure 2
The structure for the compound valerophenone is shown in Figure 2.
(c)
Interpretation:
The structure for the compound
Concept introduction:
IUPAC stands for International Union of Pure and Applied Chemistry. The IUPAC gave certain rules which are used worldwide to name the organic compounds. Due to IUPAC naming, each compound has a unique name which is different from their common name.
Answer to Problem 19.1P
The structure of
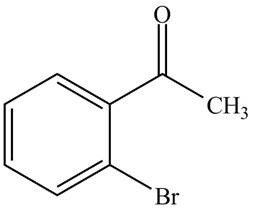
Explanation of Solution
The compound

Figure 3
The structure of the compound
(d)
Interpretation:
The structure for the compound
Concept introduction:
IUPAC stands for International Union of Pure and Applied Chemistry. The IUPAC gave certain rules which are used worldwide to name the organic compounds. Due to IUPAC naming, each compound has a unique name which is different from their common name.
Answer to Problem 19.1P
The structure of the compound
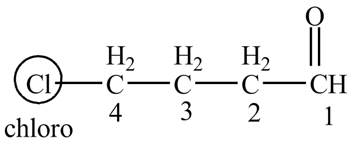
Explanation of Solution
The compound given in the question is

Figure 4
The IUPAC name of the compound is
The structure of the compound
(e)
Interpretation:
The structure for the compound
Concept introduction:
IUPAC stands for International Union of Pure and Applied Chemistry. The IUPAC gave certain rules which are used worldwide to name the organic compounds. Due to IUPAC naming, each compound has a unique name which is different from their common name.
Answer to Problem 19.1P
The structure for the compound
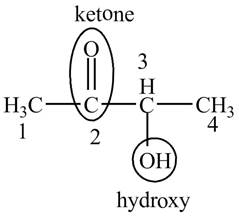
Explanation of Solution
The compound
This compound is also known as acetyl methyl carbinol. The structure of the compound is shown below.

Figure 5
The structure of the compound
(f)
Interpretation:
The structure for the compound
Concept introduction:
IUPAC stands for International Union of Pure and Applied Chemistry. The IUPAC gave certain rules which are used worldwide to name the organic compounds. Due to IUPAC naming, each compound has a unique name which is different from their common name.
Answer to Problem 19.1P
The structure for the compound
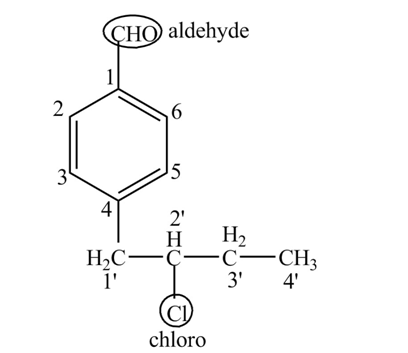
Explanation of Solution
The compound

Figure 6
The structure of the compound
(g)
Interpretation:
The structure for the compound
Concept introduction:
IUPAC stands for International Union of Pure and Applied Chemistry. The IUPAC gave certain rules which are used worldwide to name the organic compounds. Due to IUPAC naming, each compound has a unique name which is different from their common name
Answer to Problem 19.1P
The structure for the compound
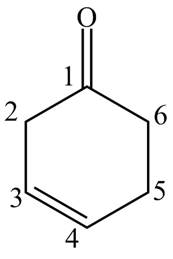
Explanation of Solution
The compound

Figure 7
The structure of the compound
(h)
Interpretation:
The structure for the compound
Concept introduction:
IUPAC stands for International Union of Pure and Applied Chemistry. The IUPAC gave certain rules which are used worldwide to name the organic compounds. Due to IUPAC naming, each compound has a unique name which is different from their common name
Answer to Problem 19.1P
The structure of the compound
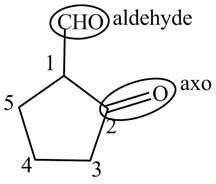
Explanation of Solution
The compound
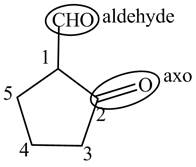
Figure 8
The structure of the compound
Want to see more full solutions like this?
Chapter 19 Solutions
Organic Chemistry
- Draw the structures of the following compounds:(a) Ethanoic acid(b) Bromopentane(c) Butanonearrow_forwardGive reasons for the following :(i) Phenol is more acidic than methanol.(ii) The C—O—H bond angle in alcohols is slightly less than the tetrahedral angle (190°28′).(iii) (CH3)3C—O—CH3 on reaction with HI gives (CH3)3C—I and CH3—OH as the main products and not (CH3)3C—OH and CH3—I.arrow_forwardDescribe how would you distinguish the following pairs, (a) Benzene and cyclohexane (b) Phenol and toluene (c) Phenol and benzoic acid (d) methanol and isopropyl alcoholarrow_forward
- (a) Give chemical tests to distinguish between the following :(i) Benzoic acid and ethyl benzoate (ii) Benzaldehyde and acetophenonearrow_forwardDraw structural formulas for these compounds. (a) 1-Bromo-2-chloro-4-ethylbenzene (b) 4-Bromo-1,2-dimethylbenzene (c) 2,4,6-Trinitrotoluene (d) 4-Phenyl-1-pentene (e) p-Cresol (f) 2,4-Dichlorophenolarrow_forwardIn each of the following reactions, two possible organic products can be formed. Draw both organic products in each case and then circle the one formed in greatest quantity in each case. HC (a) 1) NaH, 2) acid (b) CH,CH,OH (c) CH,CH,OH NH2 (d) Oarrow_forward
- 1. Draw structures corresponding to the following IUPAC names: (a) 4-Methylpentanoic acid (b) o-Hydroxybenzoic acid (c) 2,2-Dimethylpropanoyl chloride (d) trans-2-Methylcyclohexanecarboxamide (e) p-Methylbenzoic anhydride (f) p-Bromobenzonitrilearrow_forwardDraw structural formulas for these ketones. (a) Ethyl isopropyl ketone (b) 2-Chlorocyclohexanone (c) 2,4-Dimethyl-3-pentanone (d) Diisopropyl ketone (e) Acetone (f) 2,5-Dimethylcyclohexanonearrow_forwardWrite the reagent or draw structures of the starting material or organic product(s) in the following reactions. If more than one product is formed, identify the major product where possible. (a) (b) HO OH OH H2SO4 ? Cl₂ ? FeCl3arrow_forward
- Draw the structures of the following compounds:(a) tert-butylaminearrow_forwardWrite structural formulas for the following compounds (includes both old- and new-style names).(a) 2-octyne (b) ethylisopentylacetylene (c) ethynylbenzene(d) cyclohexylacetylene (e) 5-methyl-3-octyne (f) trans-3,5-dibromocyclodecynearrow_forwardGive reasons for the following: (i) p-nitrophenol is more acidic than p-methylphenol. (ii) Bond length of C—O bond in phenol is shorter than that in methanol. (iii) (CH3)3C—Br on reaction with sodium methoxide (Na+ _OCH3) gives alkene as the main product and not an ether.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





