
a)
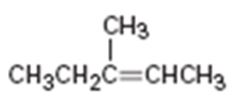
Interpretation:
Assuming Markovnikov’s rule is valid, the major alcohol product obtainable by the acid-catalyzed addition of water to 3-methyl-3-butene is to be predicted.
Concept introduction:
Markovnikov’s rule can be used to decide on the orientation in electrophilic addition reactions. According to this rule, in the addition of HX to an
To state:
The major alcohol product obtainable by the acid-catalyzed addition of water to 3-methyl-3-butene assuming that Markovnikov’s rule is valid.
b)
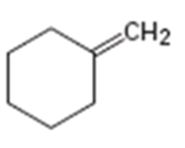
Interpretation:
Assuming Markovnikov’s rule is valid, the major alcohol product obtainable by the acid-catalyzed addition of water to methylidenecyclohexene is to be predicted.
Concept introduction:
Markovnikov’s rule can be used to decide on the orientation in electrophilic addition reactions. According to this rule, in the addition of HX to an alkene, the H attaches itself with the carbon with fewer alkyl substituents and the X attaches itself with the carbon with more alkyl substituents.
To state:
The major alcohol product obtainable by the acid-catalyzed addition of water to methylidenecyclohexene assuming that Markovnikov’s rule is valid.
c)
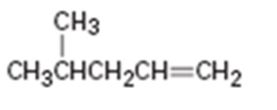
Interpretation:
Assuming Markovnikov’s rule is valid, the major alcohol product obtainable by the acid-catalyzed addition of water to 4- methyl-1-pentene is to be predicted.
Concept introduction:
Markovnikov’s rule can be used to decide on the orientation in electrophilic addition reactions. According to this rule, in the addition of HX to an alkene, the H attaches itself with the carbon with fewer alkyl substituents and the X attaches itself with the carbon with more alkyl substituents.
To state:
The major alcohol product obtainable by the acid-catalyzed addition of water to 4- methyl-1-pentene assuming that Markovnikov’s rule is valid.
Trending nowThis is a popular solution!

Chapter 7 Solutions
Organic Chemistry
- A single alkyl bromide reactant theoretically yields either of the given products, depending on the reaction conditions. Draw the structure of the alkyl bromide compound.arrow_forwardExplain the Hydroboration–oxidation two-step reaction sequence that converts an alkene to an alcohol.arrow_forwardProvide the major organic product of the following reaction. H3O', heatarrow_forward
- What two alkenes give rise to attached alcohol as the major product of acid-catalyzed hydration?arrow_forwardBriefly explain why it is important that the molecule be a b-ketoester –that is, that the ketone is two carbons away from the ester. Whatproblem(s) would arise if the ketone was three or four bonds from the ester?arrow_forwardTrue or False Considering that two carbon chains have equal number of carbons, but one has Fluorine and the other has Iodine, the one with iodine will have a higher boiling point. Mild oxidation of alkenes results to similar product as that of nucleophilic addition of water to aldehydes.arrow_forward
- We have covered several oxidants that use a multi-valent atom (Cr, Cl, S, or I) as theiractive species, going from a higher oxidation state before the oxidation to a lower oxidation state after oxidizing the alcohol. Draw the structure of the following atoms, beforeand after the oxidation of an alcohol to a ketone or aldehyde. How many bonds to oxygendoes each atom have before and after the oxidation?(a) the Cr in chromic acid (b) the Cl in sodium hypochlorite(c) the S in the Swern oxidation (d) the I in the DMP reagent(e) the carbinol C in the alcohol that is oxidizedarrow_forwardWe have covered several oxidants that use a multi-valent atom (Cr, Cl, S, or I) as their active species, going from a higher oxidation state before the oxidation to a lower oxidation state after oxidizing the alcohol. Draw the structure of the following atoms, before and after the oxidation of an alcohol to a ketone or aldehyde. How many bonds to oxygen does each atom have before and after the oxidation? (a) the I in the DMP reagent (b) the carbinol C in the alcohol that is oxidizedarrow_forwardOrganic Chemistry Incorrect Separation Scheme Cannot be hand-drawn *see attached for the incorrect separation scheme provided. The top of the separation scheme shows what other compound is mixed with your molecule (2,6-dimethyloct-2-ene). Assume for the purposes of this assignment that both compounds are solid at room temperature. Also assume that both compounds are soluble in ether, except ionic compounds. The goal of the separation is the isolate each of the two compounds from the mixture. Below the incorrect separation scheme write a discussion of this incorrect scheme identifying all of the mistakes in the separation scheme. Keep in mind that there will be more than one mistake in the scheme. For each mistake, give a detailed, scientific explanation of why it is incorrect.arrow_forward

 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

